Magnetically-controllable delivery system for therapeutic agents
a delivery system and therapeutic agent technology, applied in the field of magnetization controllable delivery systems, can solve the problems of neo-intimal growth or proliferation, major limitation of in-stent restenosis (the re-closing of the vessel), and the ability of known drug delivery vehicles to localize high concentrations of drugs using minimally invasive techniques, etc., to achieve the effect of increasing the degree of magnetization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
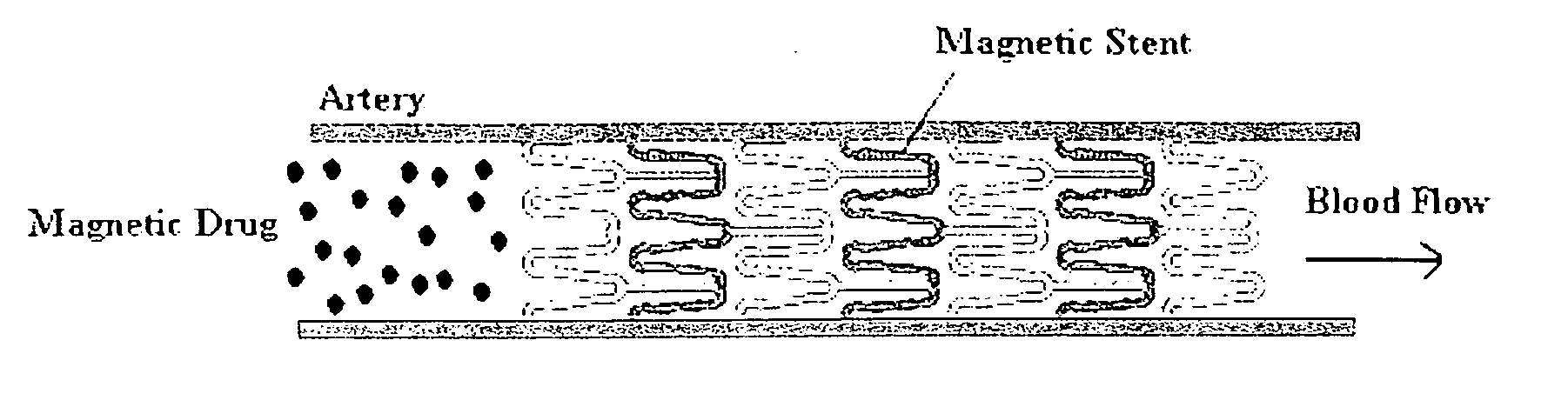
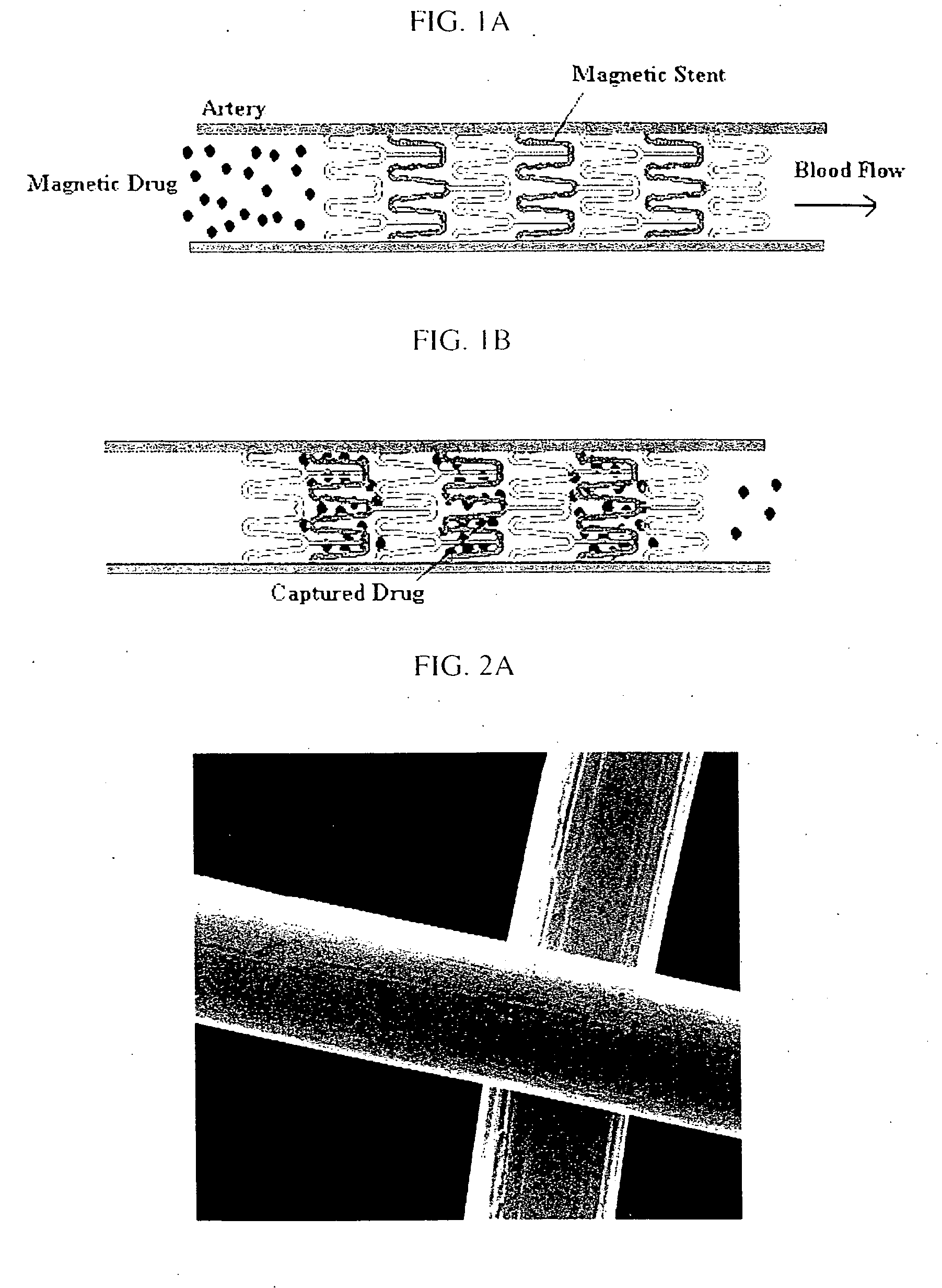
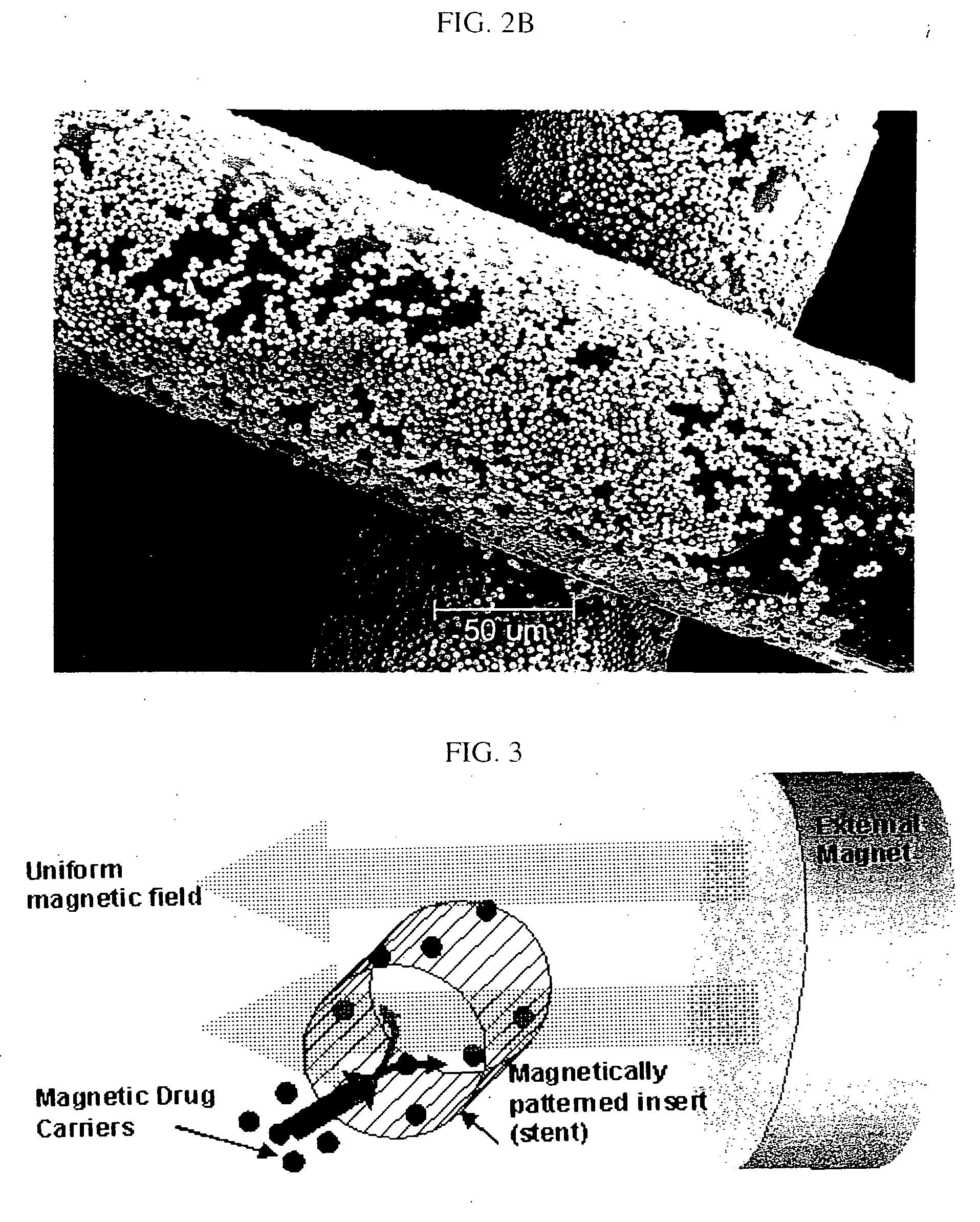
[0048] The invention was driven by a desire to develop a system capable of targeted delivery of magnetizable particles to a location within a body. Inventors have discovered that magnetizable particles are attracted to regions of the strongest magnetic field gradients and devised a two source system that produces strong and highly localized field gradients inside the body utilizing (1) a magnetizable object implanted in a body as an internal source of a magnetic gradient and (2) an external source of a magnetic field. Unlike the one source systems known in the prior art, this invention utilizes two sources of magnetic influence for targeted delivery of magnetizable particles to areas on the magnetizable object implanted in a body and / or in the near proximity thereto.
[0049] Accordingly, the invention provides a magnetic delivery system for delivering a magnetizable particle to a location in a body, the device comprising a magnetizable object implanted in the body, wherein the magnet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com