Methods of treating skin conditions using inhibitors of the CD2/LFA-3 interaction
a technology of cd2/lfa3 and skin conditions, which is applied in the direction of antibody medical ingredients, instruments, peptide/protein ingredients, etc., can solve the problems of inadequate current therapies for the above mentioned skin diseases, and achieve the effects of less immunosuppression, less general toxicity, and increased t cell activation and abnormal antigen presentation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Subjects
[0111] Six adult patients participated in the investigation. Informed consent was obtained after Internal Review Board approval of the protocol. All patients satisfied the major diagnostic criteria for psoriasis, namely chronic papulosquamous plaques of characteristic morphology and distribution. -The intermittent use of topical corticosteroids was common among these patients but was discontinued 2 weeks prior to entry-into the study. A group of healthy volunteers with no history of psoriasis or other skin disease was utilized as the normal control group.
Preparation of Epidermal Cell Suspensions
[0112] Skin biopsy specimens were obtained from both normal and lesional skin by using a keratome. The specimens were submerged in Dulbecco's phosphate buffered saline (“PBS”); (Gibco Labs, Grand Island, N.Y.) containing 50 units / ml dispase (Collaborative Research, Bedford, Mass.). The specimens were then incubated at 4° C. for 18 hours and the epidermis removed from the remainin...
example 2
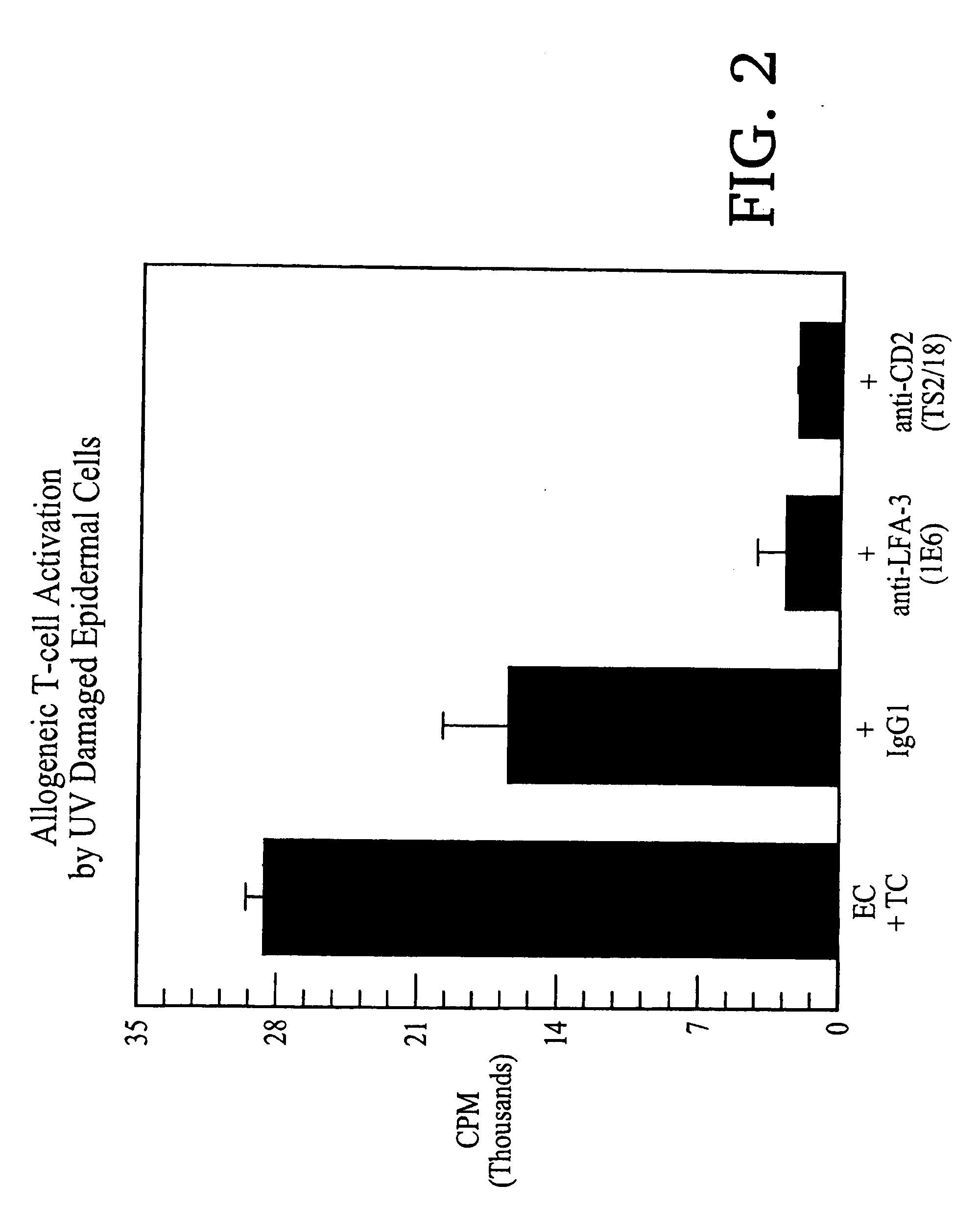
Subject
[0125] One adult subject participated in this investigation. Informed consent was obtained after Internal Review Board approval of the protocol. The minimal dose of UV B from a bank of fluorescent bulbs (FS 40) required to induce skin erythema in the subject was determined prior to the study. A moderate sunburn (4 minimal erythemal doses) was then administered to the left buttock, which 3 days later was the source of UV damaged skin. Skin from the right buttock, which was unburned, was utilized for the control.
Preparation of Epidermal Cell Suspensions
[0126] Skin biopsy specimens were obtained from both normal and sunburned skin by using a keratome. Epidermal cell suspensions were prepared from these specimens using substantially the same protocol as in Example 1.
Isolation and Deletion of T cells
[0127] Peripheral blood mononuclear cells (“MNC”) were isolated from heparinized blood of another person, using Ficoll-Hypaque® (Pharmacia) density gradient centrifugation acco...
example 3
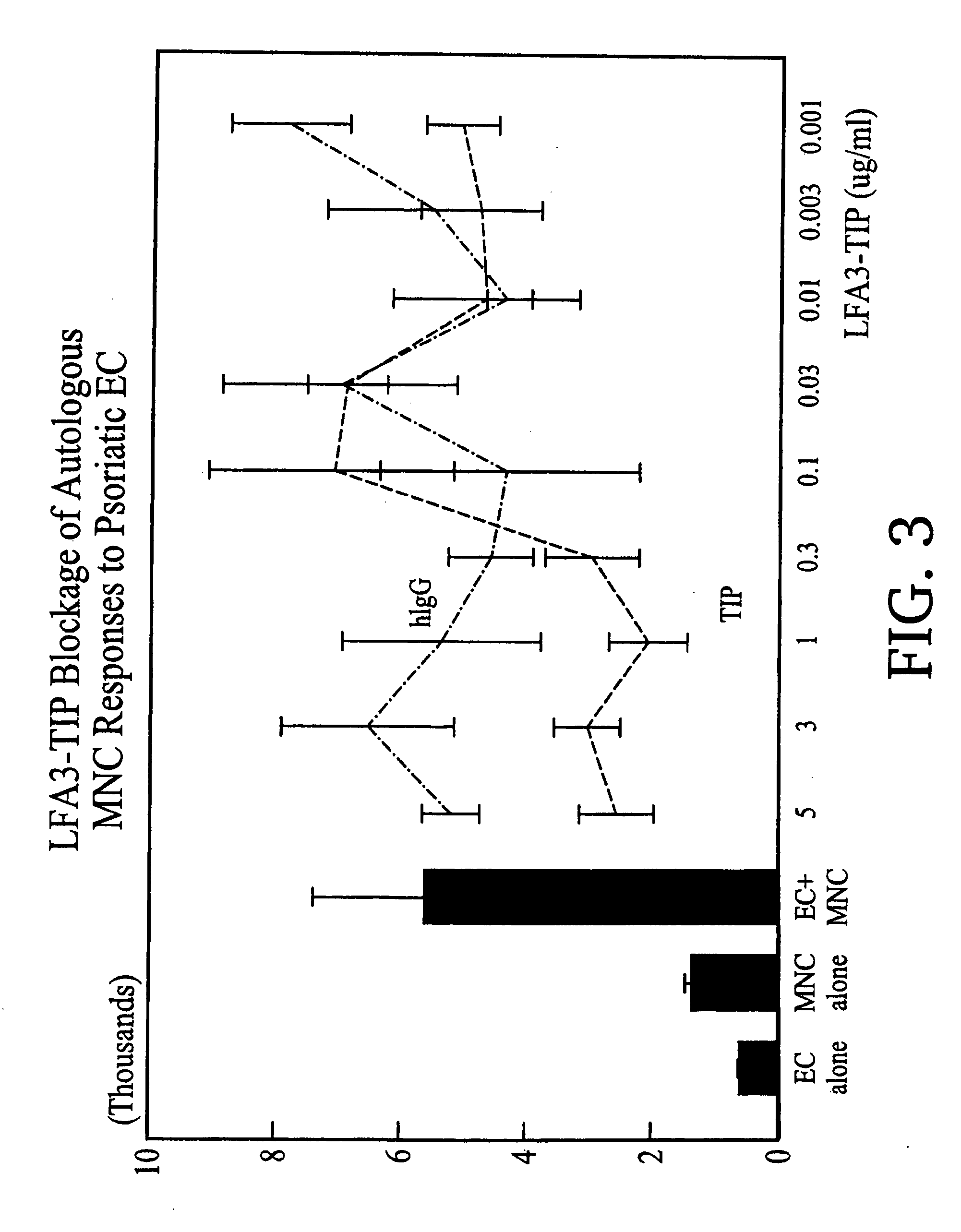
Preparation of LFA3TIP
[0132] LFA3TIP, a fusion protein comprised of the first extracellular domain of LFA-3 fused to the hinge, CH2 and CH3 regions of human IgG1 was constructed as described in Miller, GT et al. (1993) J. Exp. Med.178:211, hereby incorporated by reference. LFA3TIP was purified from culture medium of transfectant CHO (chinese hamster ovary) cell lines by absorption to Protein-A Sepharose 4B® (Pharmacia) and eluted with 50 mM glycine, 250 mM NaCl (pH.3.0). Fractions containing protein were pooled and subjected to gel filtration on Superose-6 (Pharmacia) in phosphate buffered saline (PBS). Peak fractions were pooled and analyzed for purity on 12% reducing and non-reducing SDS-PAGE.
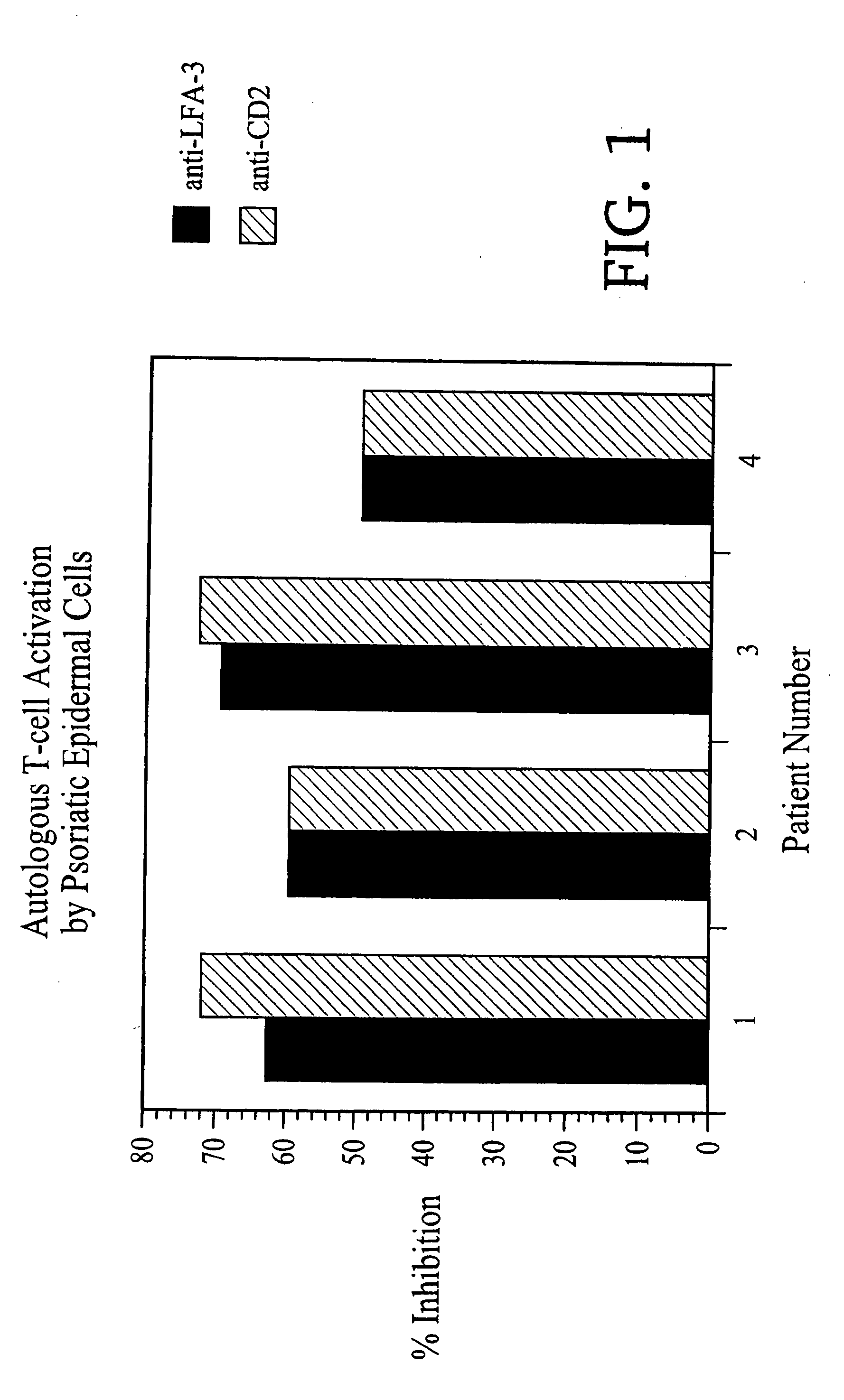
1. LFA3TIP Results in a Decrease of the Proliferative Response of Autologous Mononuclear Cells to Lesional Psoriatic Epidermal Cells
[0133] The ability of LFA3T1P to inhibit the response of autologous mononuclear cells (MNC) to lesional psoriatic epidermal cells (EC) in suspension was-det...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com