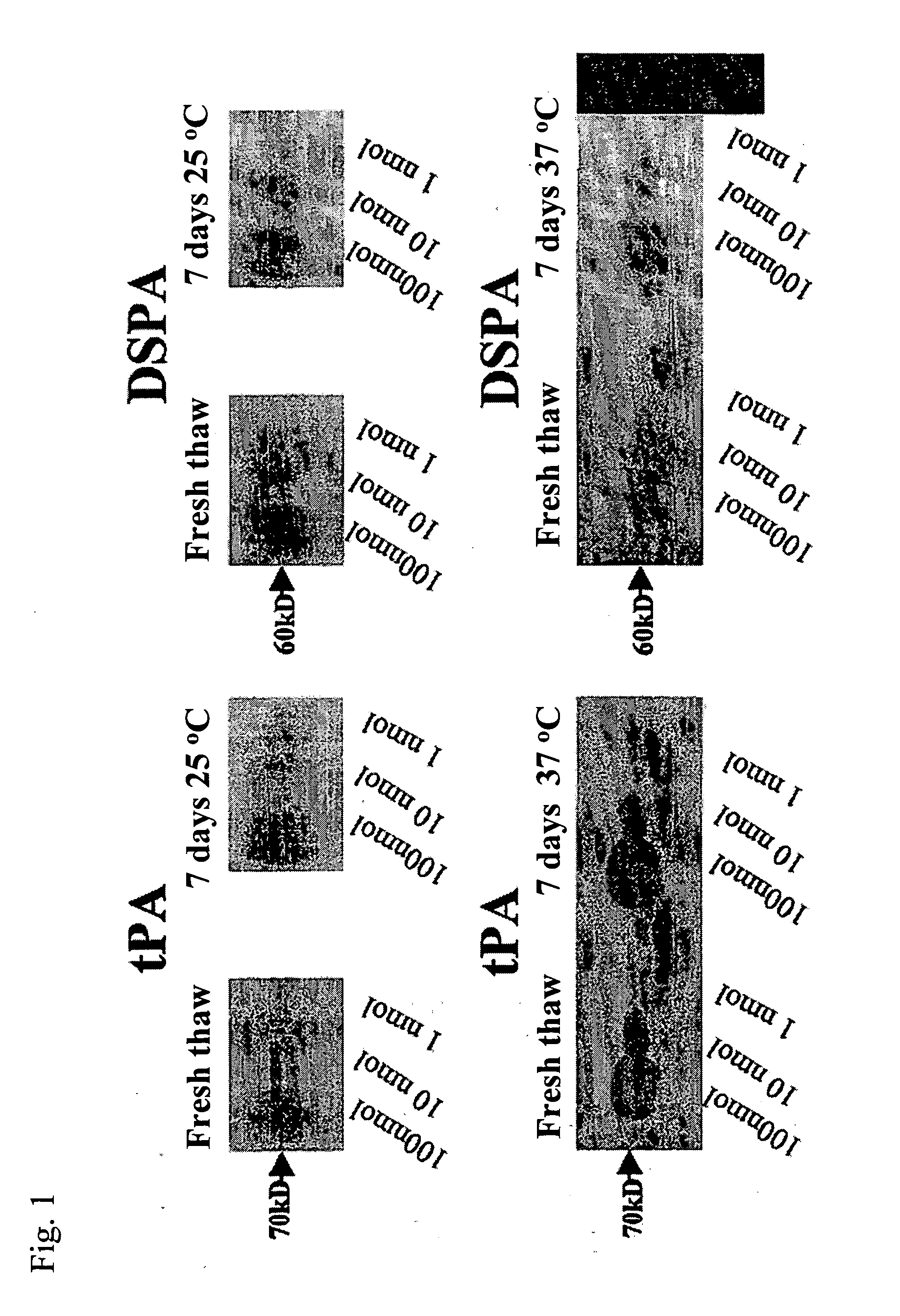
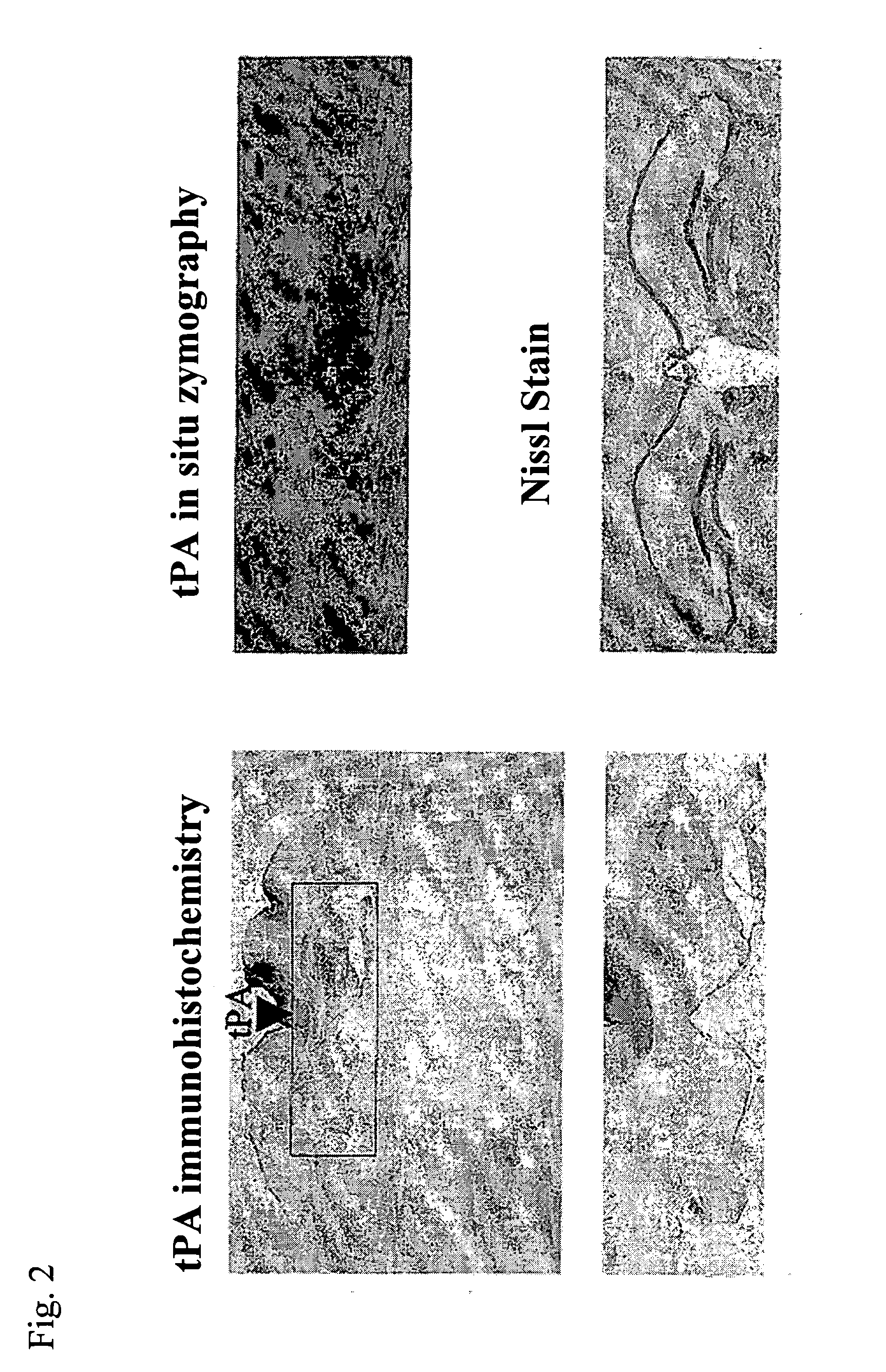
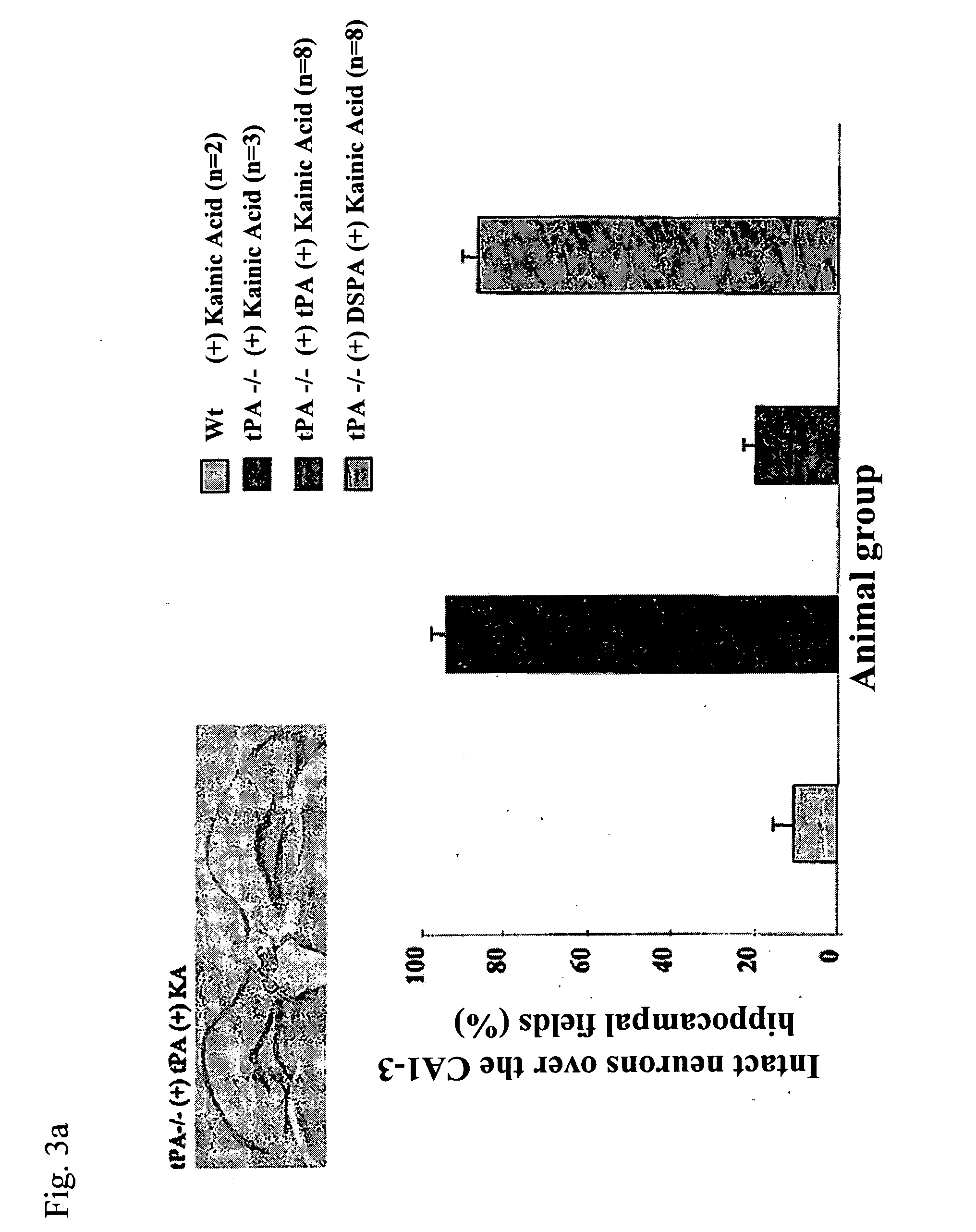
Non-neurotoxic plasminogen activating factors for treating of stroke
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0056] In a preferred embodiment t-PA is used showing point mutations in the positions 420 to 423. If these residues are substituted by directed mutagenesis the fibrin dependency of t-PA is increased by a factor up to 61,000 (K Song-Hua et al.). Song-Hua et al. examined the point mutations L420A, L420E, S421G, S421E, P422A, P422G, P422E, F423A and F423E. These publications are fully incorporated by reference for disclosure of the use according to the invention.
[0057] According to a further advantageous embodiment a modified tissue plasminogen activator with an amino acid sequence according to SEQ ID No. 1 (FIG. 10) is used. This modified t-PA differs from the wild type t-PA by the exchange of the hydrophobic amino acids in the position 420 to 423 in the autolysis loop as follows: His420, Asp421, Ala422 and Cys423. This t-PA preferentially contains a phenyl alanine at the position 194. Further the position 275 can be occupied by glutamate. Advantageously the position 194 is occupied...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com