Immunomodulating polymers
a polymer and immunomodulator technology, applied in the field of immunomodulators and methods, can solve the problems of recurring sepsis in patients, time-consuming and costly procedures, and prolonged hospitalization, and achieve the effects of reducing postoperative surgical adhesion formation, promoting allograft survival, and suppressing the igg antibody respons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Sources of Bacteria Isolation and Modification of Polysaccharides and Animal Model for Intraabdominal Sepsis
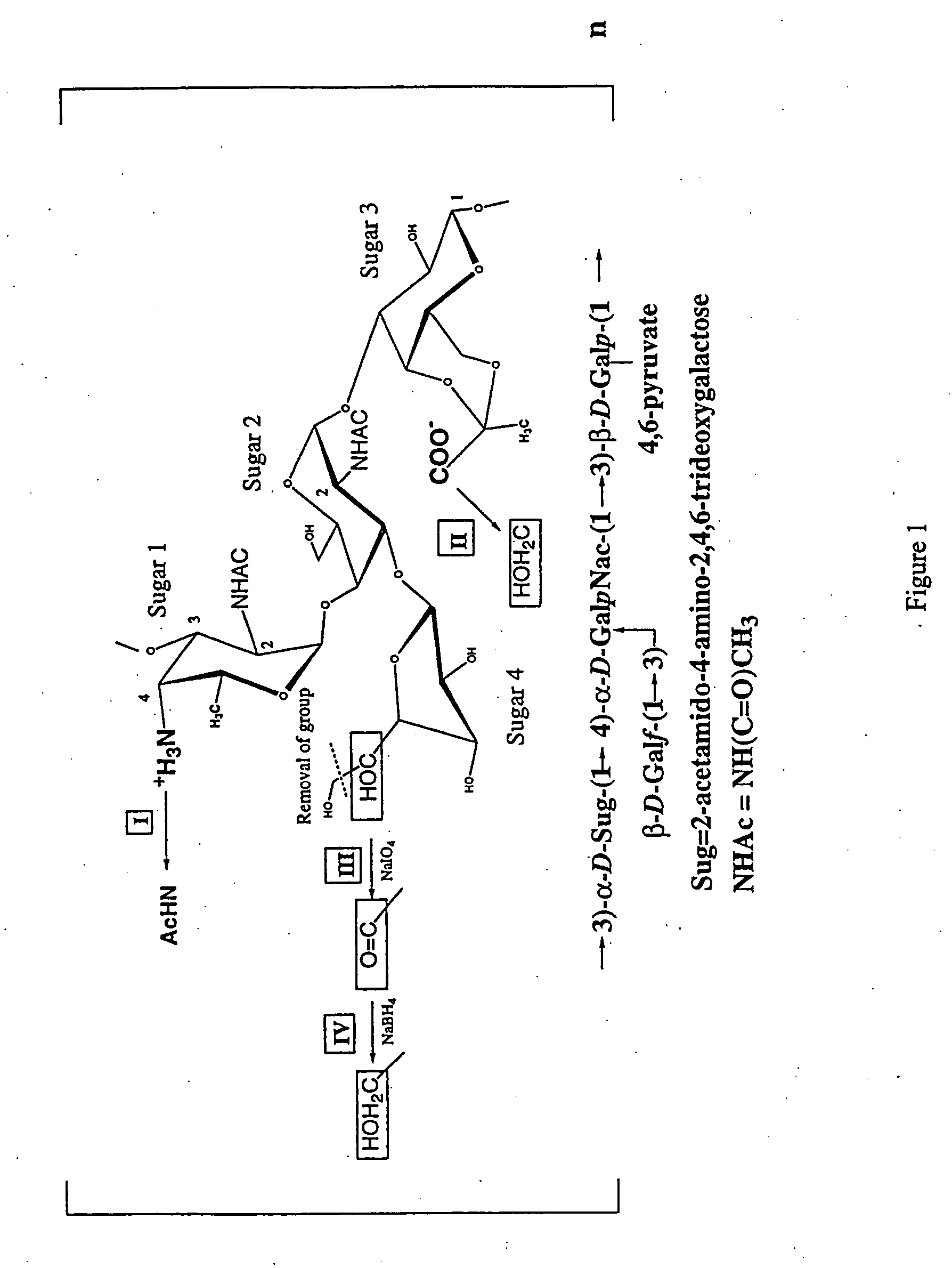
[0155]B. fragilis NCTC 9343 and ATCC 23745 were originally obtained from the National Collection of Type Cultures (London, England) or the American Type Culture Collection (Bethesda, Md.). Microorganisms were stored at −80° C. in peptone-yeast or brain heart infusion broth until used, and grown anaerobically as previously described. Pantosti et al. Infect Immun 59:2075 (1991). The CPC from B. fragilis NCTC 9343 or ATCC 23745 was isolated by hot phenol / water extraction and subsequent purification of PS A performed as previously described. Tzianabos, A et al. J Biol Chem 267:18230 (1992).
[0156] The S. pneumoniae type 1 capsular polysaccharide (CP) and other pneumococcal polysaccharides were obtained from the ATCC (MD).
[0157] Chemical modifications of polysaccharides to produce molecules with altered charges have been described previously. Taylor, R et al. Biochemistry 11:1383...
example 2
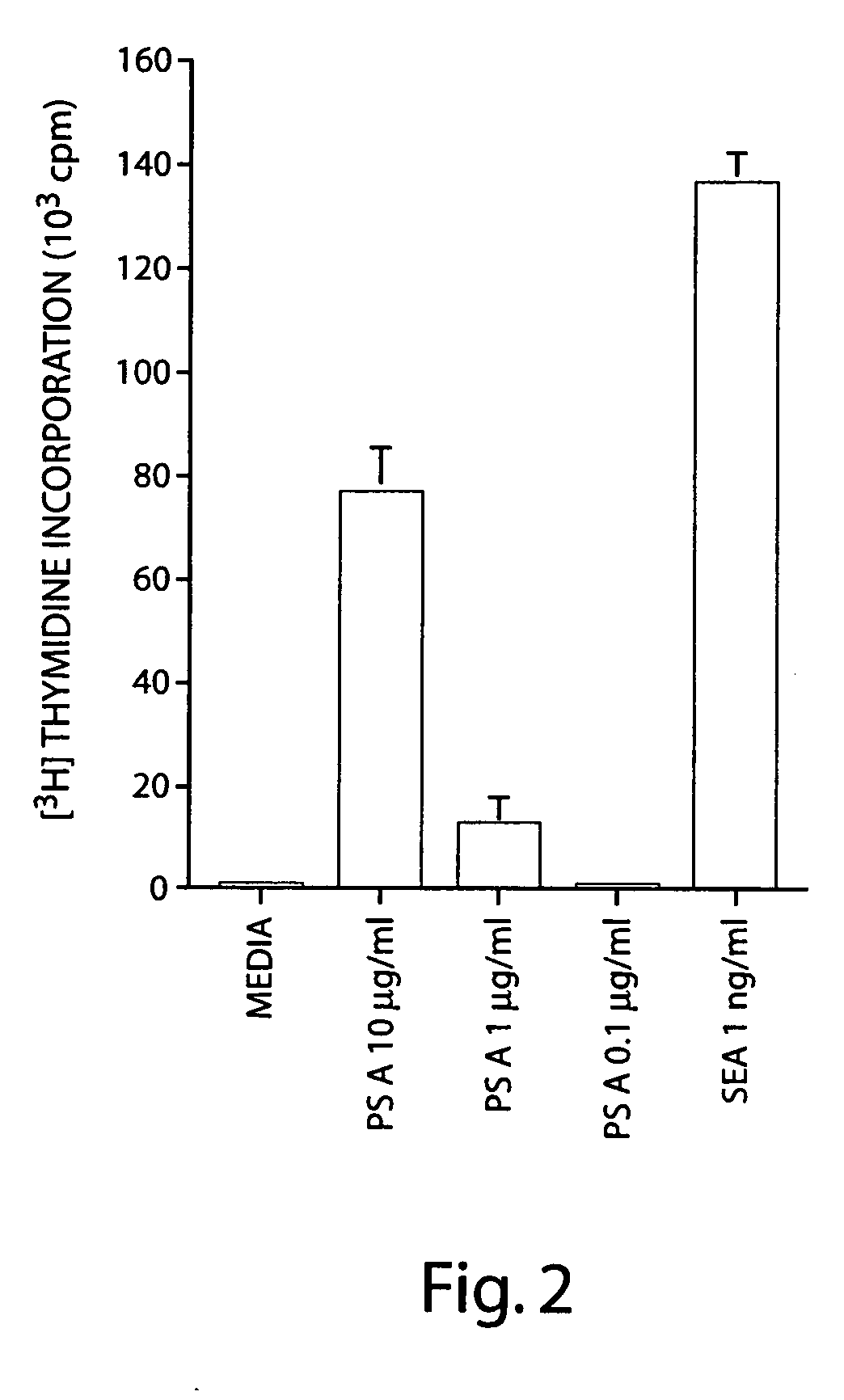
T Cell Activation by PS A Depends on Charge Motif
[0160] The ability of B. fragilis PS A to elicit a protective host response that is dependent on T cells suggested an interaction between PS A and this cell type. Thus experiments were performed to determine whether PS A activates T cells in vitro.
[0161] T cell proliferation assays were performed on cells obtained from human leukopacs (discarded white cells from anonymous platelet donors). Mononuclear cells were separated by ficoll-hypaque sedimentation to eliminate red cells and polymorphonuclear leukocytes. The mononuclear layer, which consisted of T cells, B cells, and mononuclear cells, was depleted of B cells and monocytes by passage over nylon wool column. A portion of these cells was saved prior to placement on nylon wool and were used as autologous feeder cells following irradiation with 6.4 krads with a cesium source for 4.8 min. Nylon passed cells, which were greater than 98% CD3 positive (as determined by FACS analysis) w...
example 3
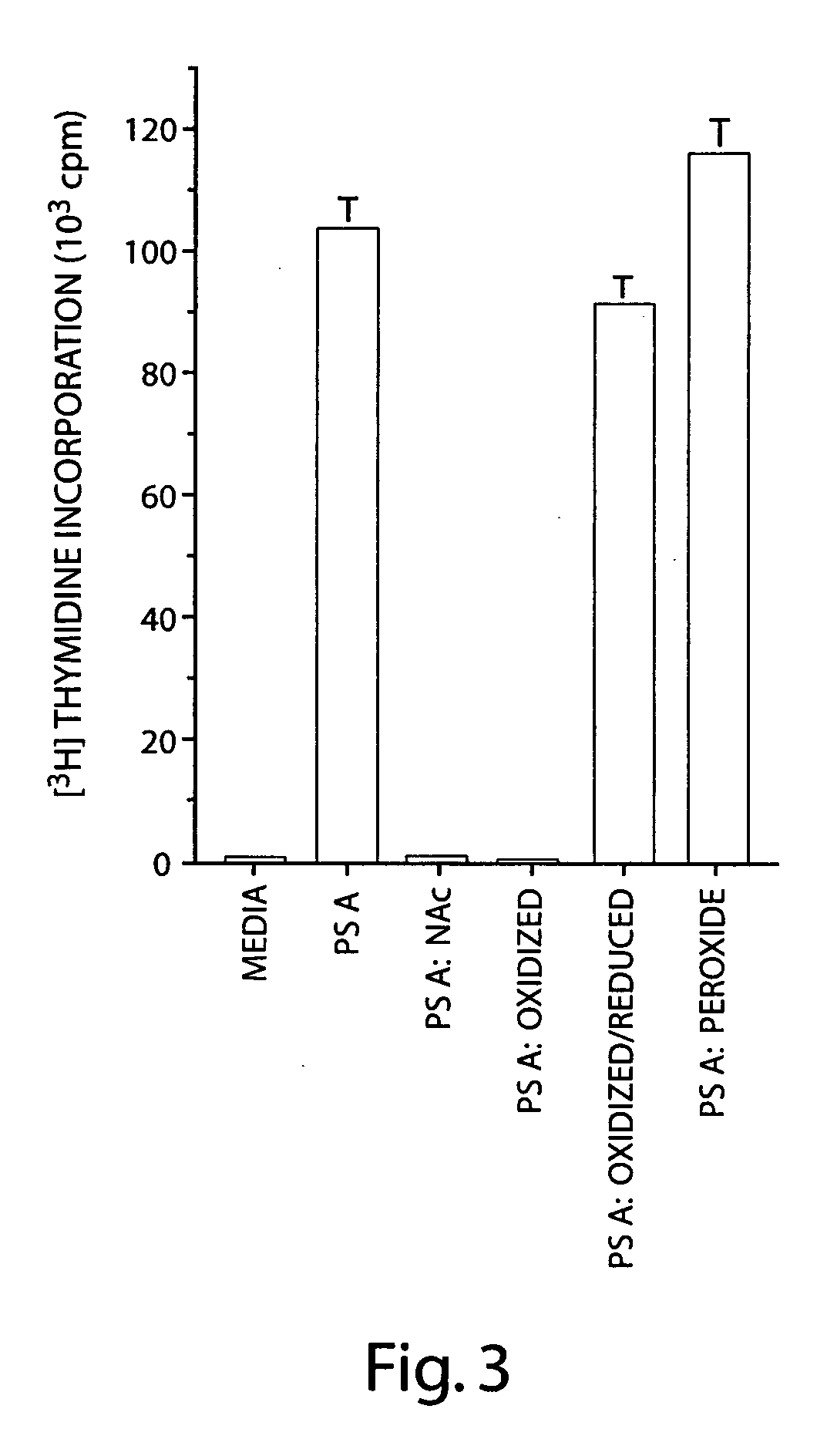
Characterization of Zwitterionic Polymer Charge Motif Responsible for T Cell Activation
[0167] This example examines whether another bacterial polysaccharide with a charge motif similar to PS A could activate T cells in vitro. Streptococcus pneumoniae type 1 capsular polysaccharide (CP) is among the few naturally occurring polysaccharides that have oppositely charged groups. Lindberg, B et al. Carbohydr Res 78:111 (1980). The type 1 CP is a trisaccharide repeating unit that has the same sugar residue with a positively charged free amino group (2-acetamido-4-amino-2,4,6-trideoxygalactose residue) that occurs in PS A. In addition, the type 1 CP has two galacturonic acid residues containing negatively charged carboxyl groups per repeating unit. In previous studies, we have demonstrated that like PS A, the type 1 CP also protects animals against abscess formation. Tzianabos, A O et al. Infect Immun 62:4881 (1994). In addition, this protective activity is also dependent on the presence o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com