Spinal ligament modification devices
a technology of spinal ligaments and modifications, applied in the field of minimally invasive methods and devices for treating spinal disorders, can solve the problems of spinal instability of spine patients, back and leg pain, weakness and numbness of legs, and conservative treatment options that fail frequently, so as to prevent the leakage of cerebrospinal fluid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
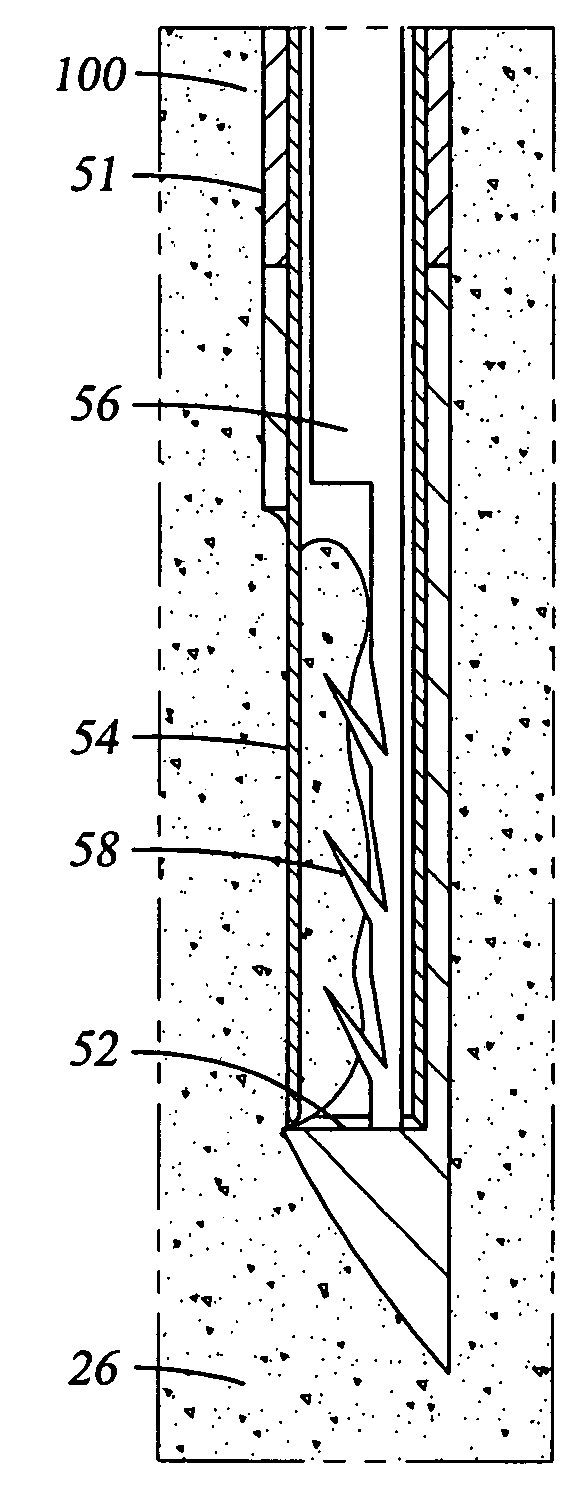
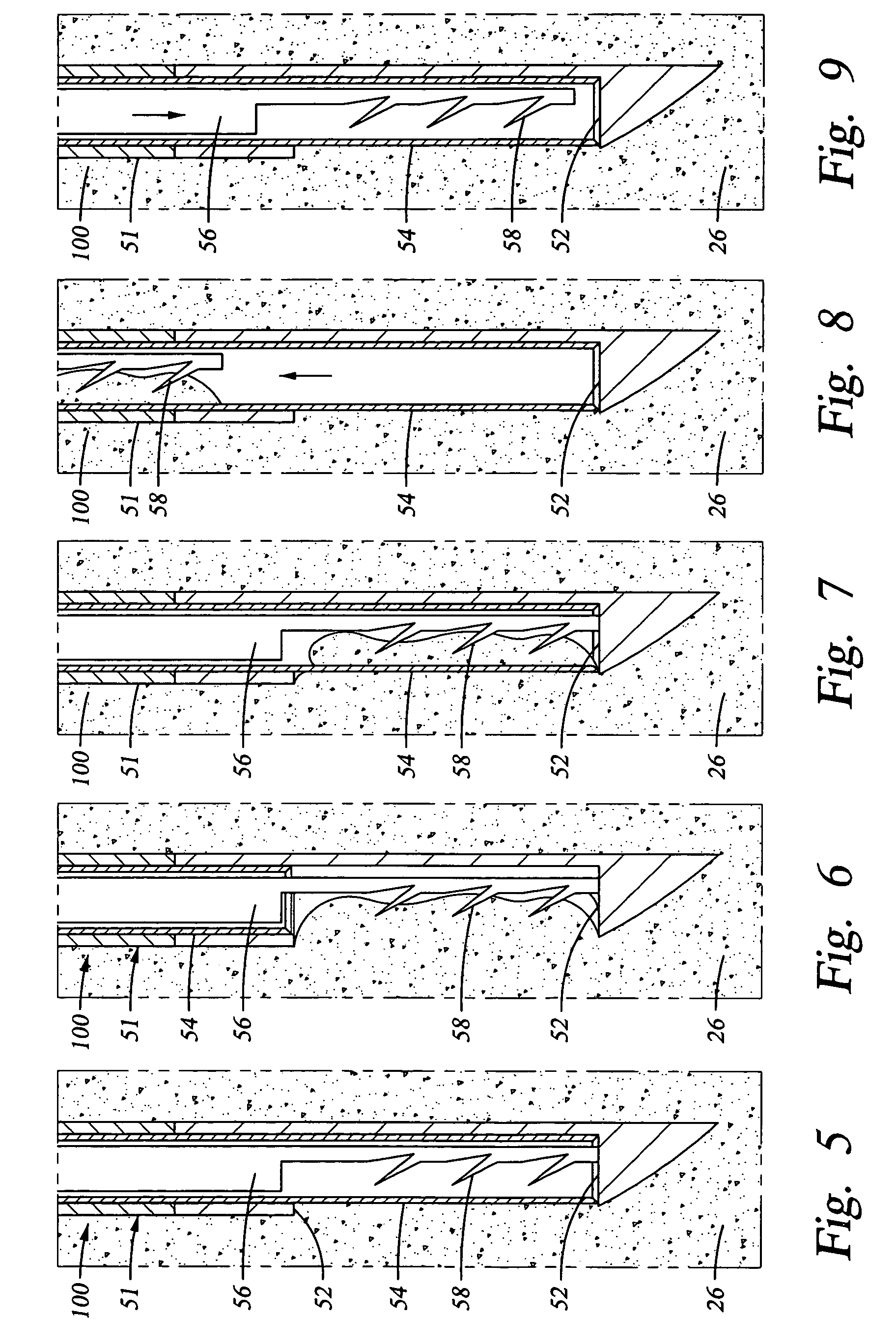
[0046] The epidural space is the space between the ligamentum flavum and the thecal sac. This space is filled with blood vessels and fat. The nerves contained within the thecal sac are normally surrounded by cerebrospinal fluid (CSF). When the ligamentum flavum hypertrophies, the blood vessels that supply the nerves of the cauda equina are compressed. This results in ischemic pain termed spinal claudication. The nerve roots may also be compressed resulting in back and / or leg pain.
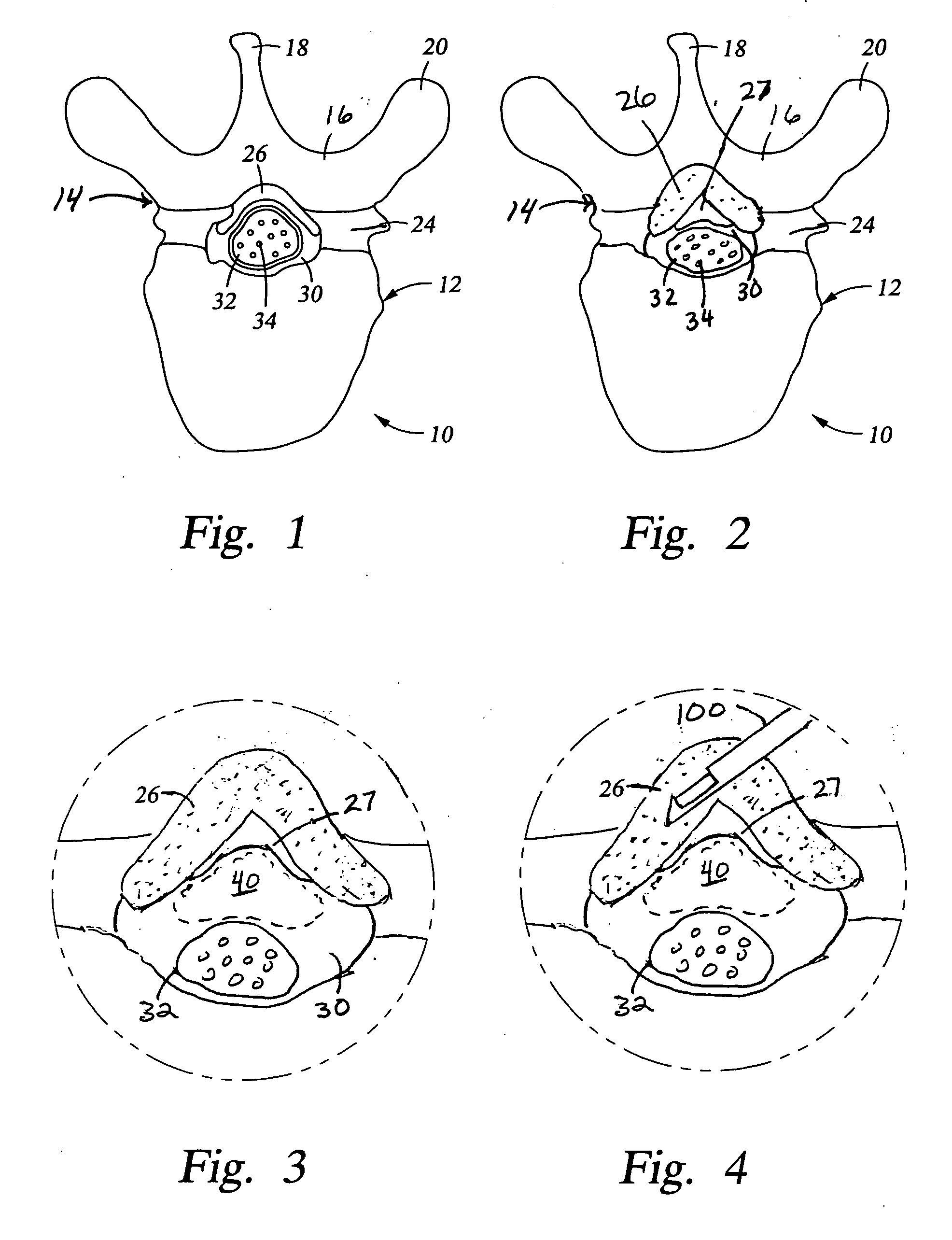
[0047] Referring again to FIG. 1, the posterior border of the normal epidural space 30 is formed by the normally thin ligamentum flavum 26 and posterior epidural fat (not shown). Ligamentum flavum 26 extends from the lamina above the interspinous space to the lamina below the interspinous space. The dural sleeve (thecal sac) 32 contains nerve roots 34 surrounded by cerebrospinal fluid. The nerve roots 34 normally comprise only a small proportion of the thecal sac volume.
[0048] In FIG. 2, spinal stenosis i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com