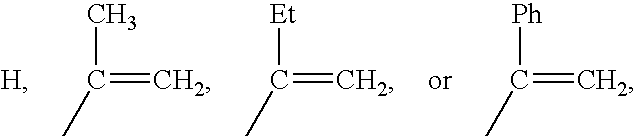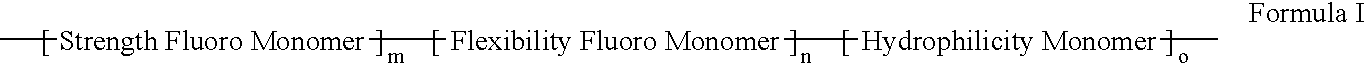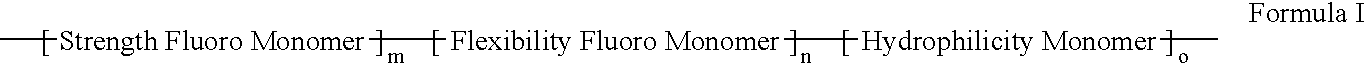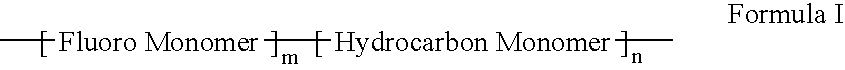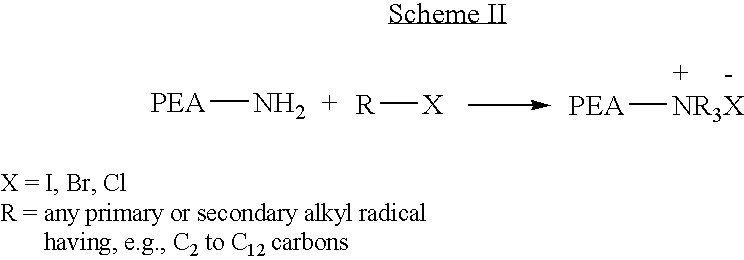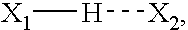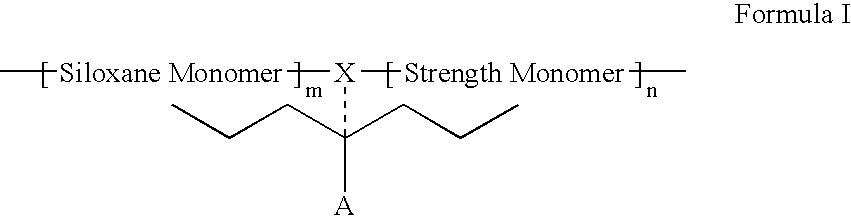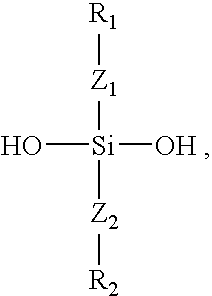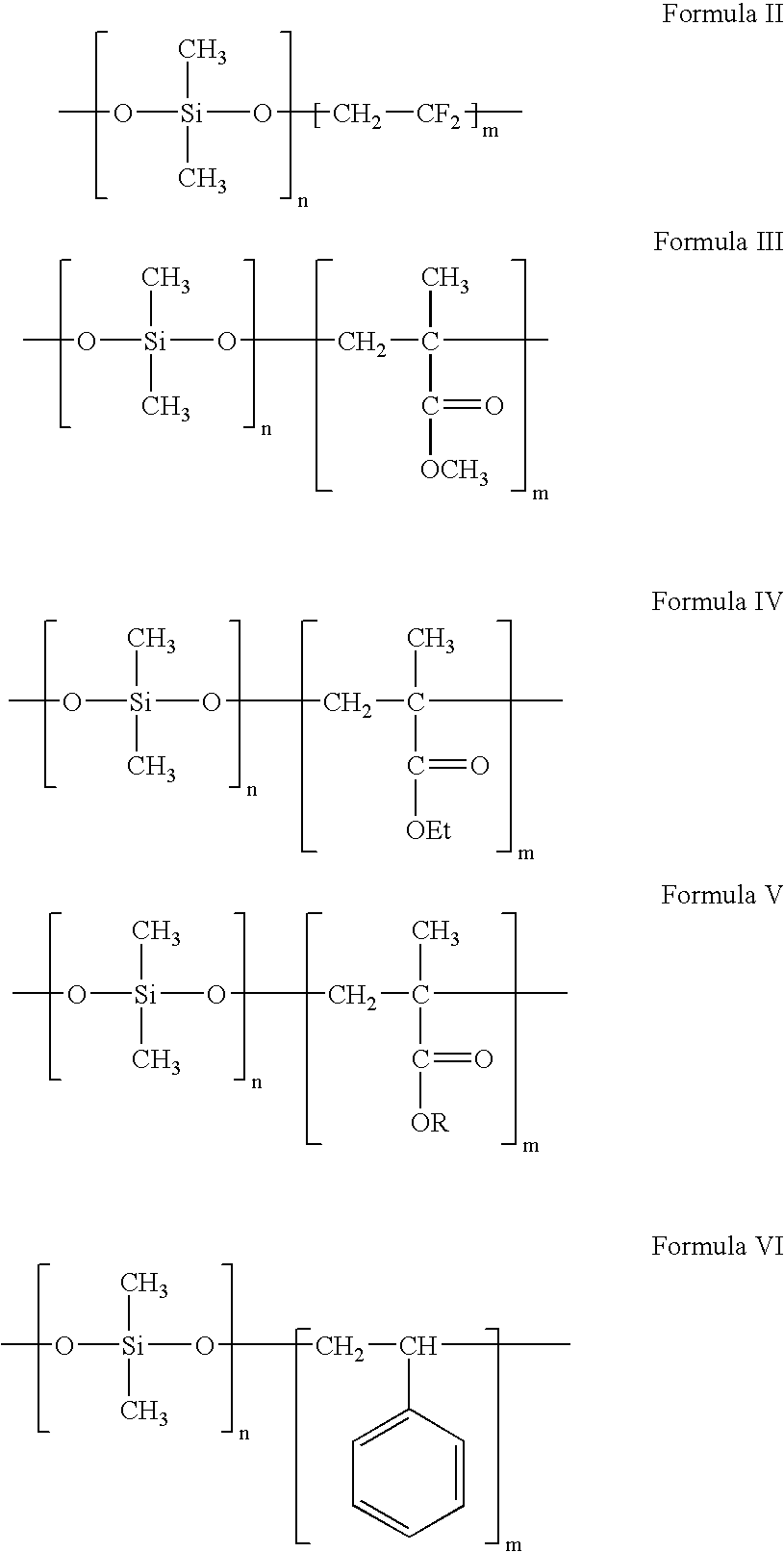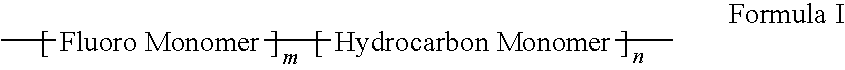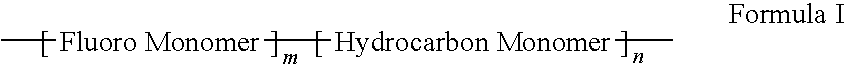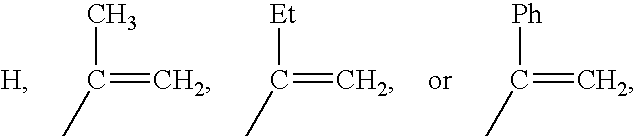Patents
Literature
45 results about "Spinal claudication" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Spinal or neurogenic claudication is not due to lack of blood supply, but rather it is caused by nerve root compression or stenosis of the spinal canal, usually from a degenerative spine, most often at the "L4-L5" or "L5-S1" level.
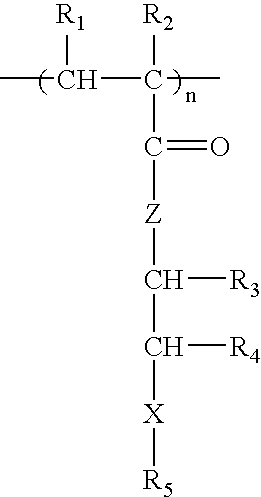
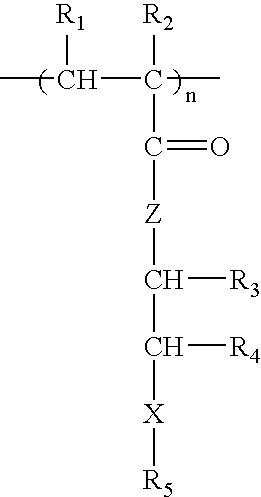
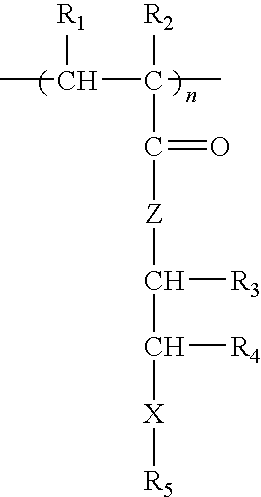
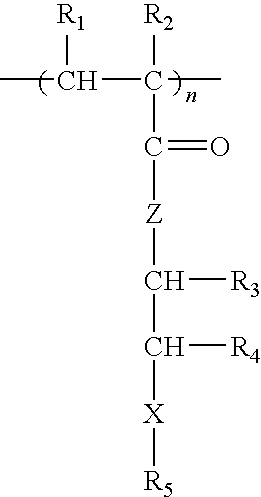
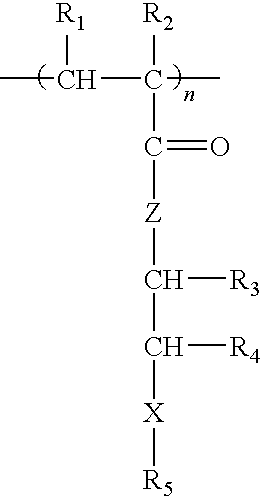
Methacrylate copolymers for medical devices
A polymer of hydrophobic monomers and hydrophilic monomers is provided. It is also provided a polymer blend that contains the polymer and another biocompatible polymer. The polymer or polymer blend and optionally a biobeneficial material and / or a bioactive agent can form a coating on an implantable device such as a drug delivery stent. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
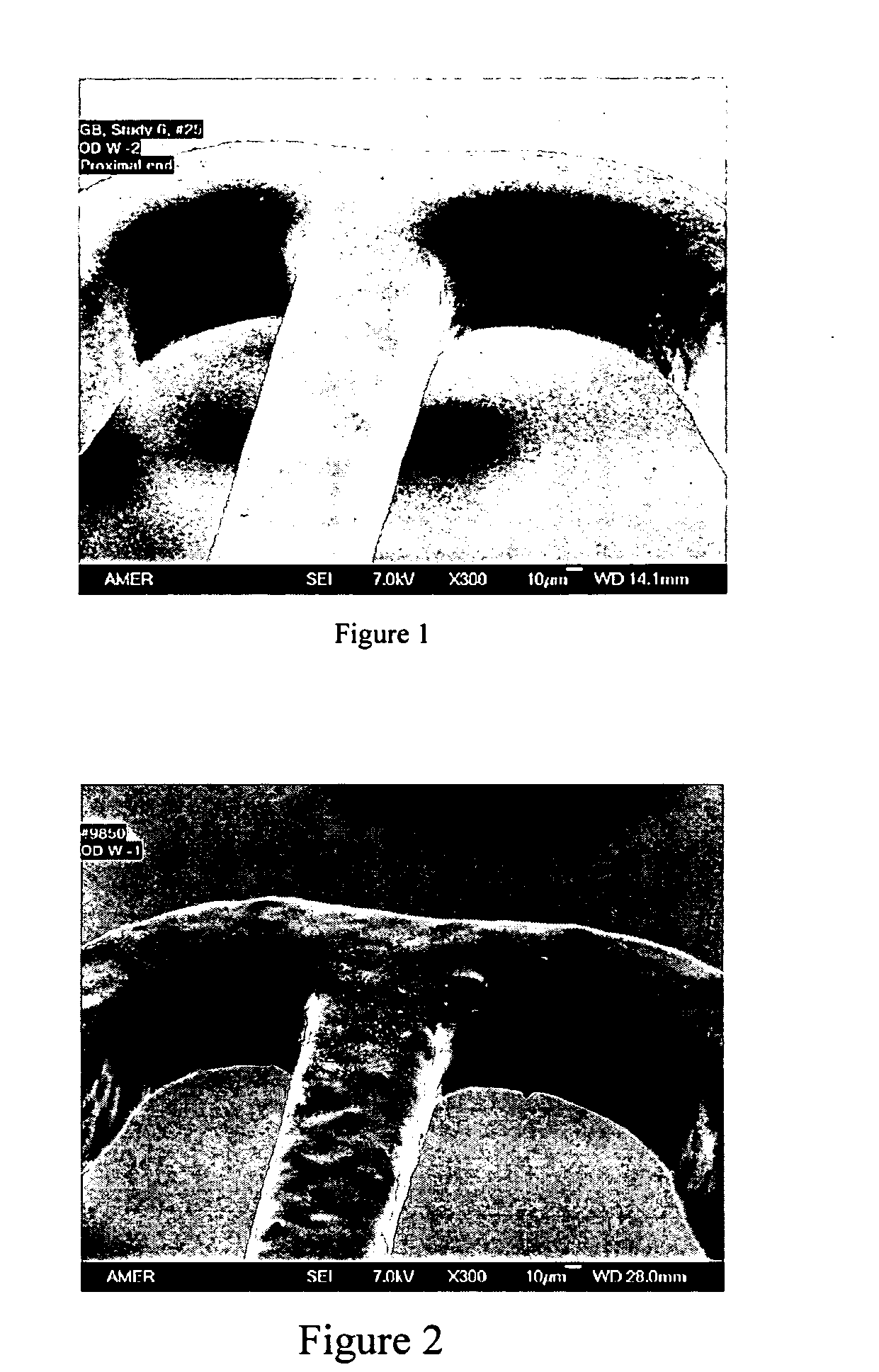
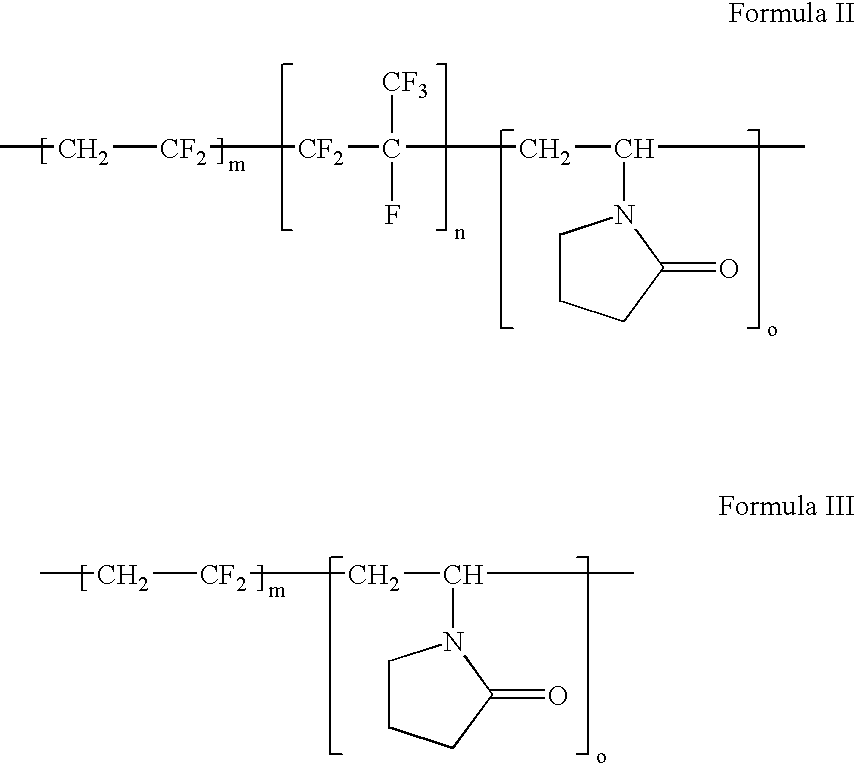
Polymers of fluorinated monomers and hydrophilic monomers
ActiveUS20060047095A1Improve propertiesProvide flexibilityFibre treatmentSurgeryDiseasePolymer science
A polymer of fluorinated monomers and hydrophilic monomers is provided. It is also provided a polymer blend that contains a polymer of fluorinated monomers and another biocompatible polymer. The polymer of fluorinated monomers or polymer blend described herein and optionally a bioactive agent can form a coating on an implantable device such as a drug-delivery stent. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
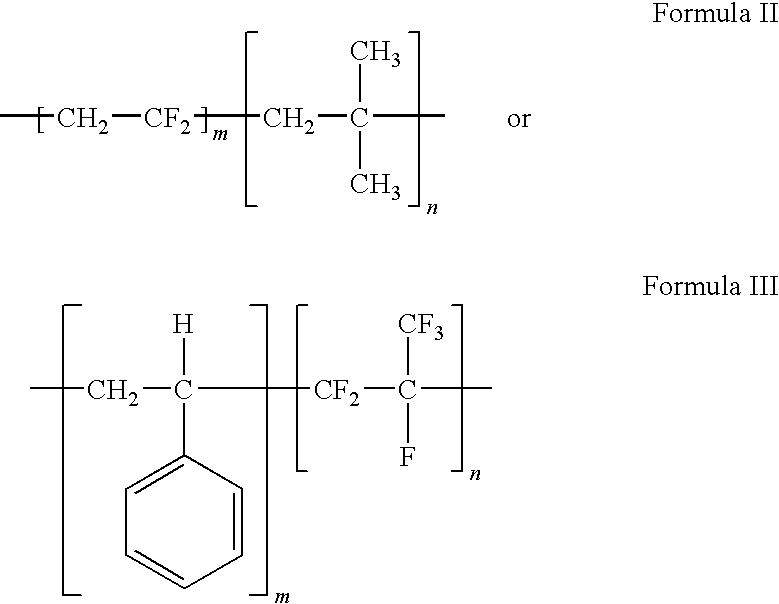
Polymers of fluorinated monomers and hydrocarbon monomers
InactiveUS20060134165A1Provide mechanical strengthGive flexibilityStentsSurgeryAbnormal tissue growthPolymer science
A polymer of fluorinated monomers and hydrocarbon monomers is provided. It is also provided a polymer blend that contains a polymer formed of fluorinated monomers and hydrocarbon monomers and another biocompatible polymer. The polymer or polymer blend described herein and optionally a bioactive agent can form an implantable device such as a stent or a coating on an implantable device such as a drug-delivery stent, which can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
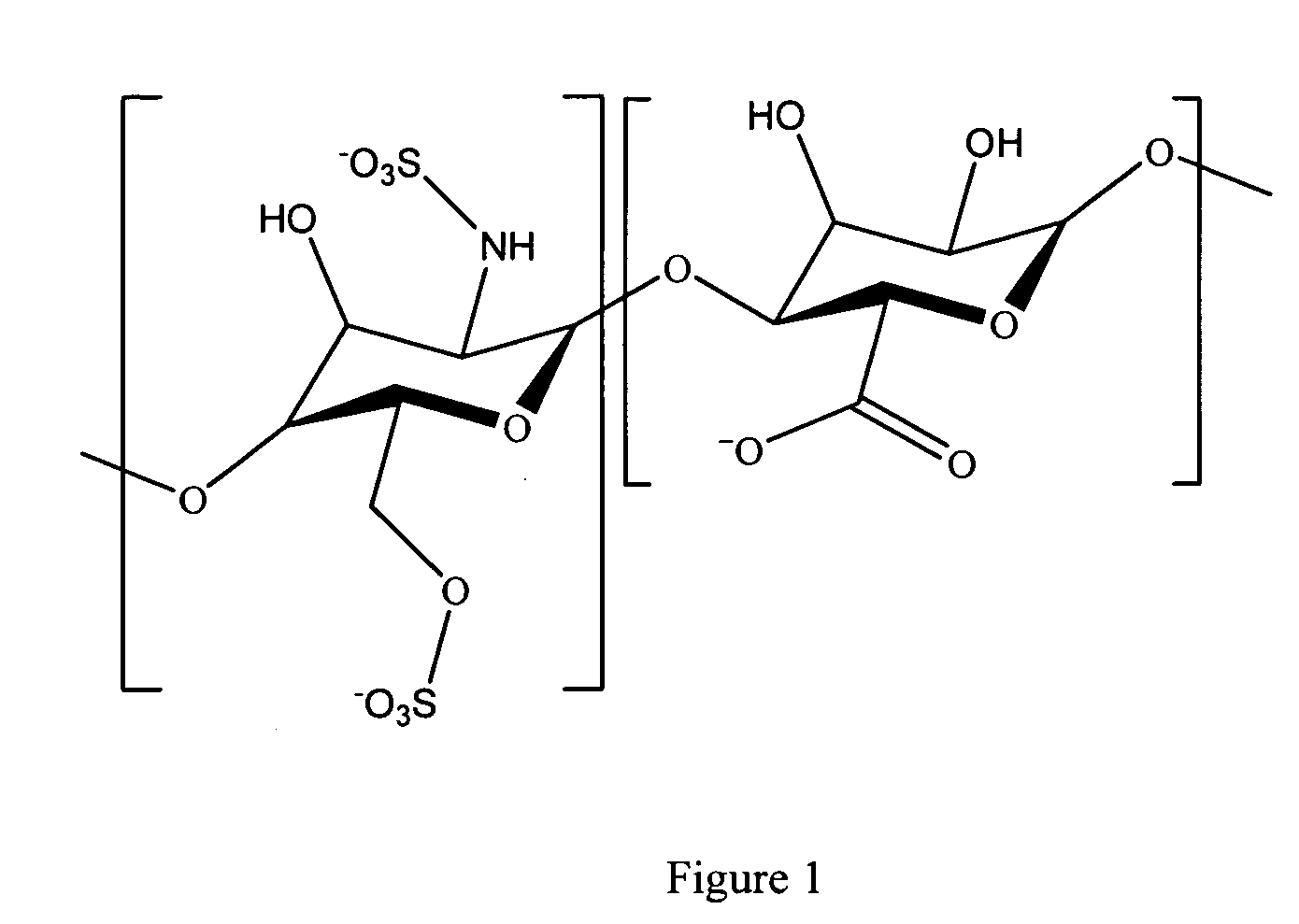
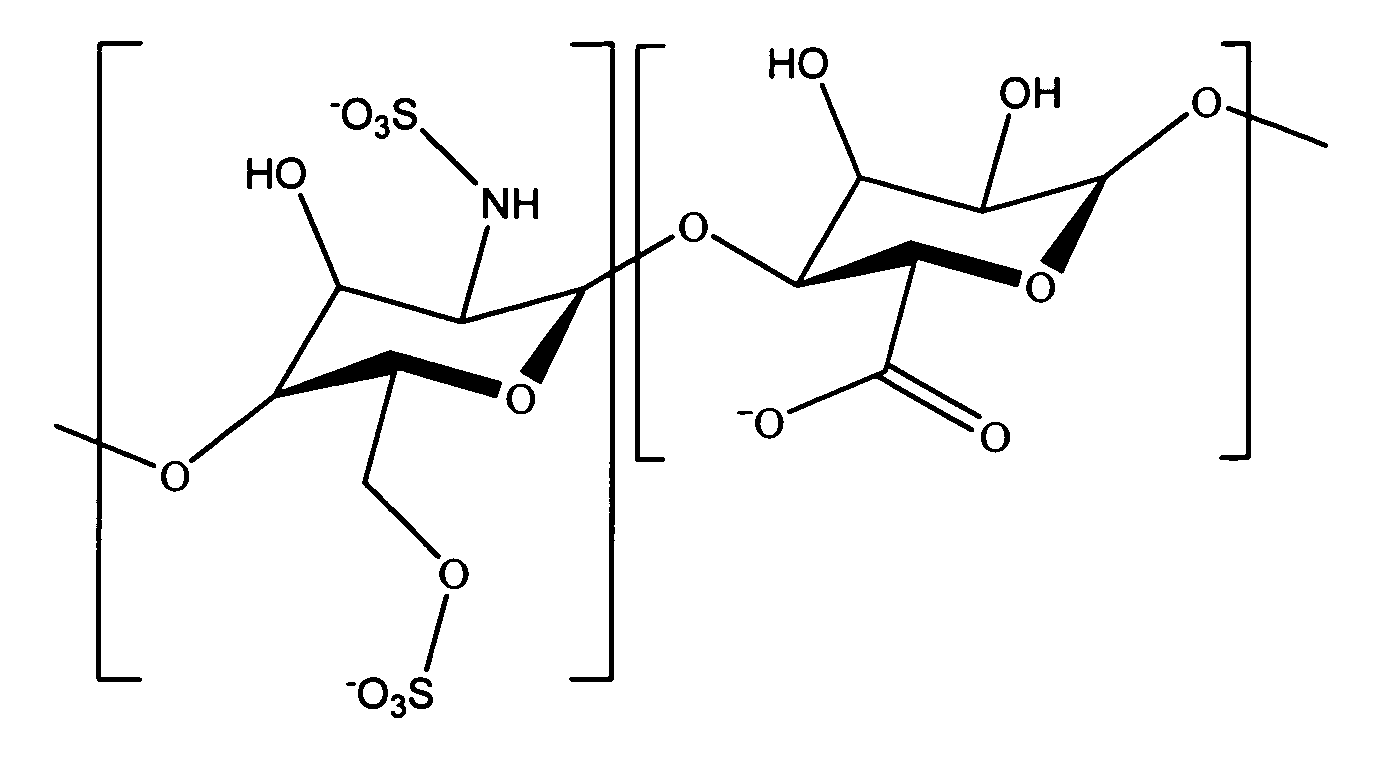
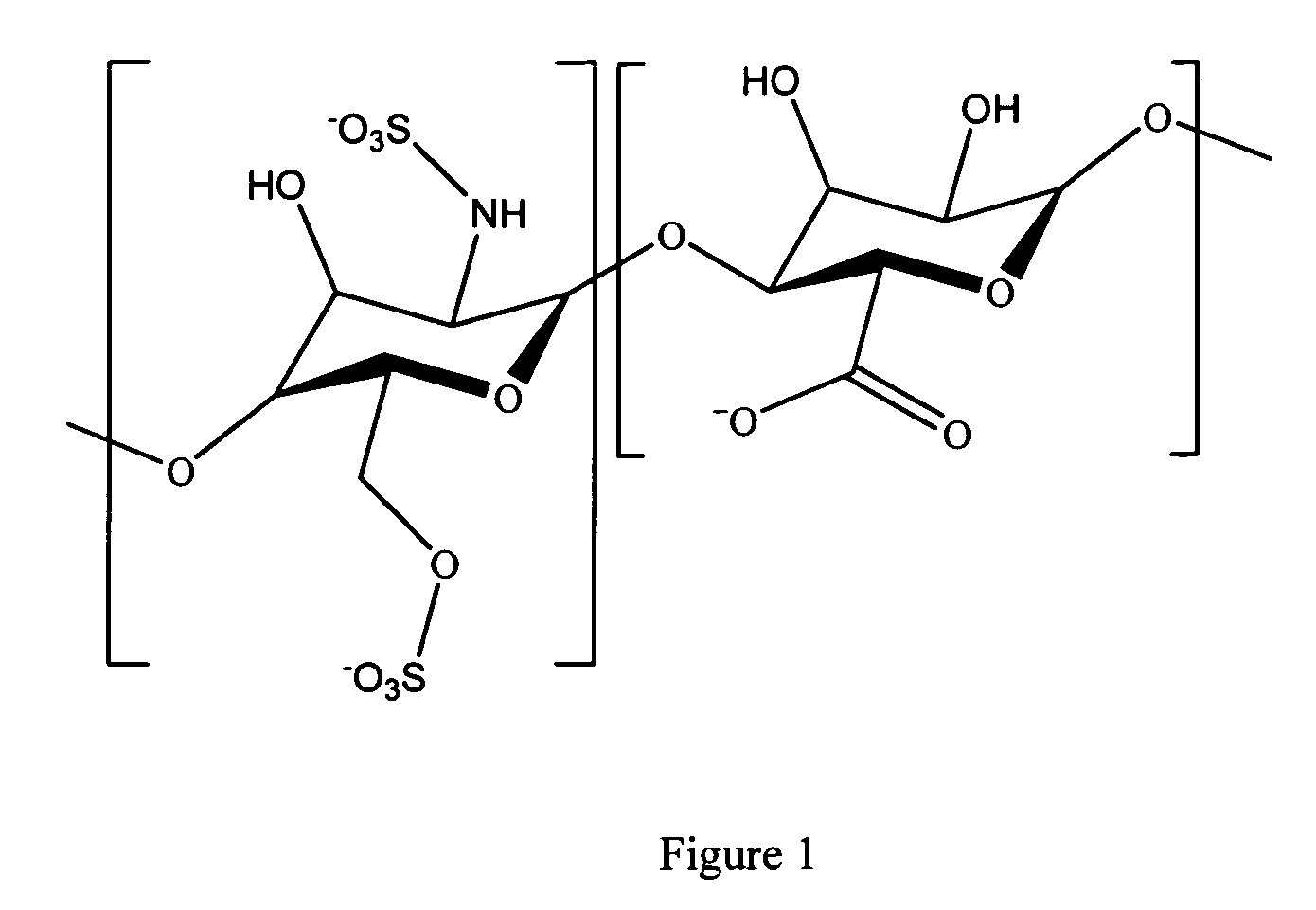
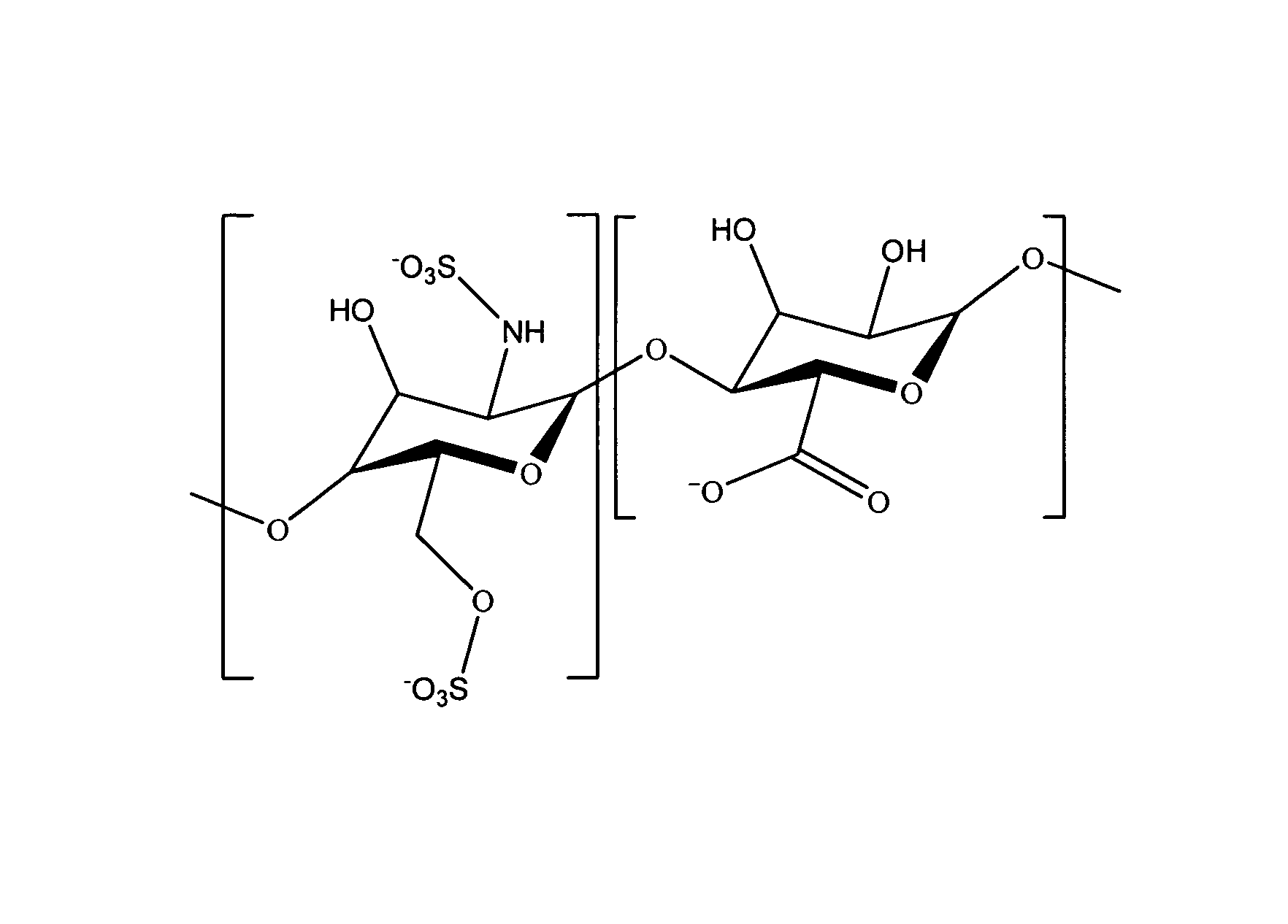
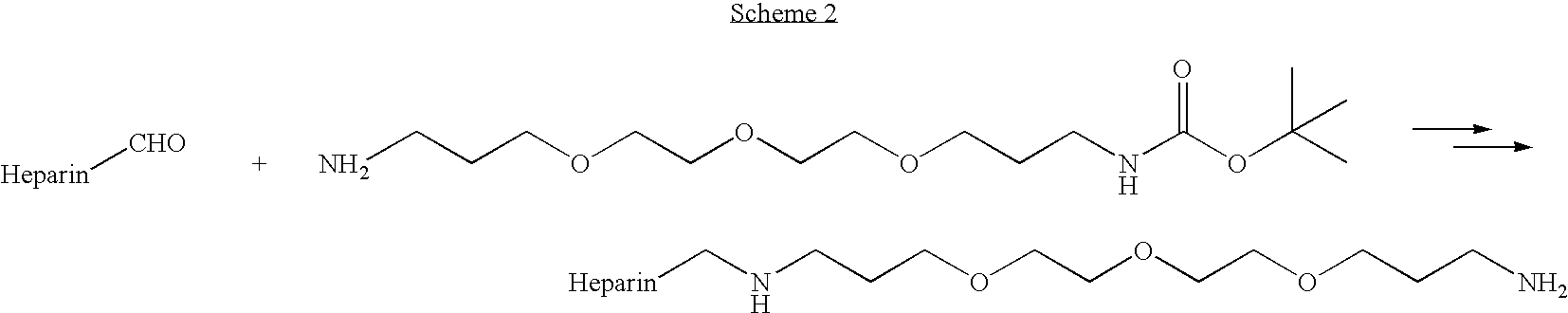
Antifouling heparin coatings
A medical device comprising a coating thereon comprising a biocompatible polymer and heparin is provided herein. Heparin is coupled with the biocompatible polymer via a spacer having a grouping that renders a binding site of the heparin molecule accessible by a binding protein. The medical device can be implanted in a human being for the treatment of a disease such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Beneficial effects of increasing local blood flow
InactiveUS20110028548A1Increase oxygenationImprove tissue nutritionBiocidePeptide/protein ingredientsArginineNitric oxide
Owner:STRATEGIC SCI & TECH
Plasticizers for coating compositions
A biocompatible plasticizer useful for forming a coating composition with a biocompatible polymer is provided. The coating composition may also include a biobeneficial polymer and / or a bioactive agent. The coating composition can form a coating on an implantable device. The implantable device can be used to treat or prevent a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
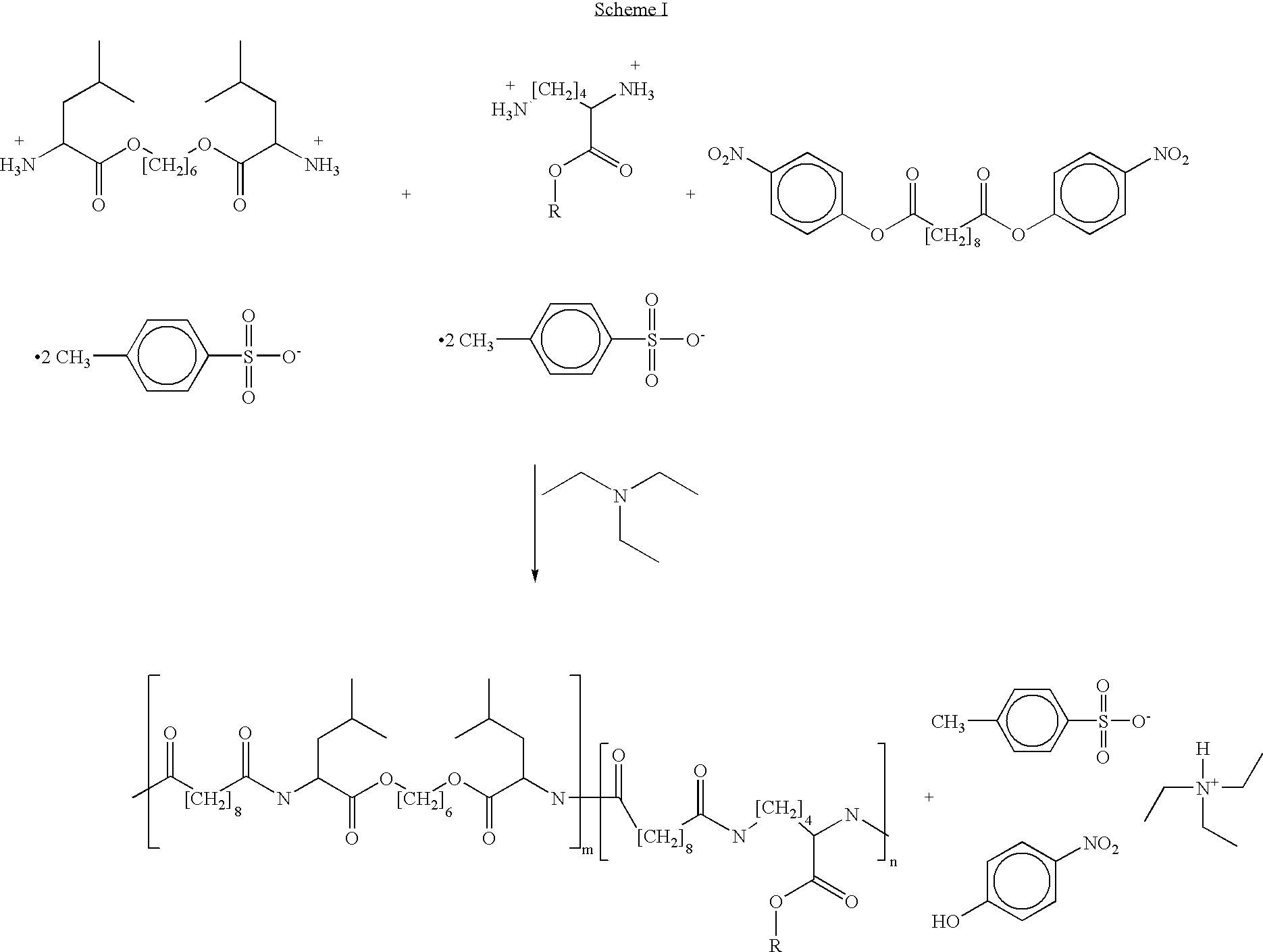
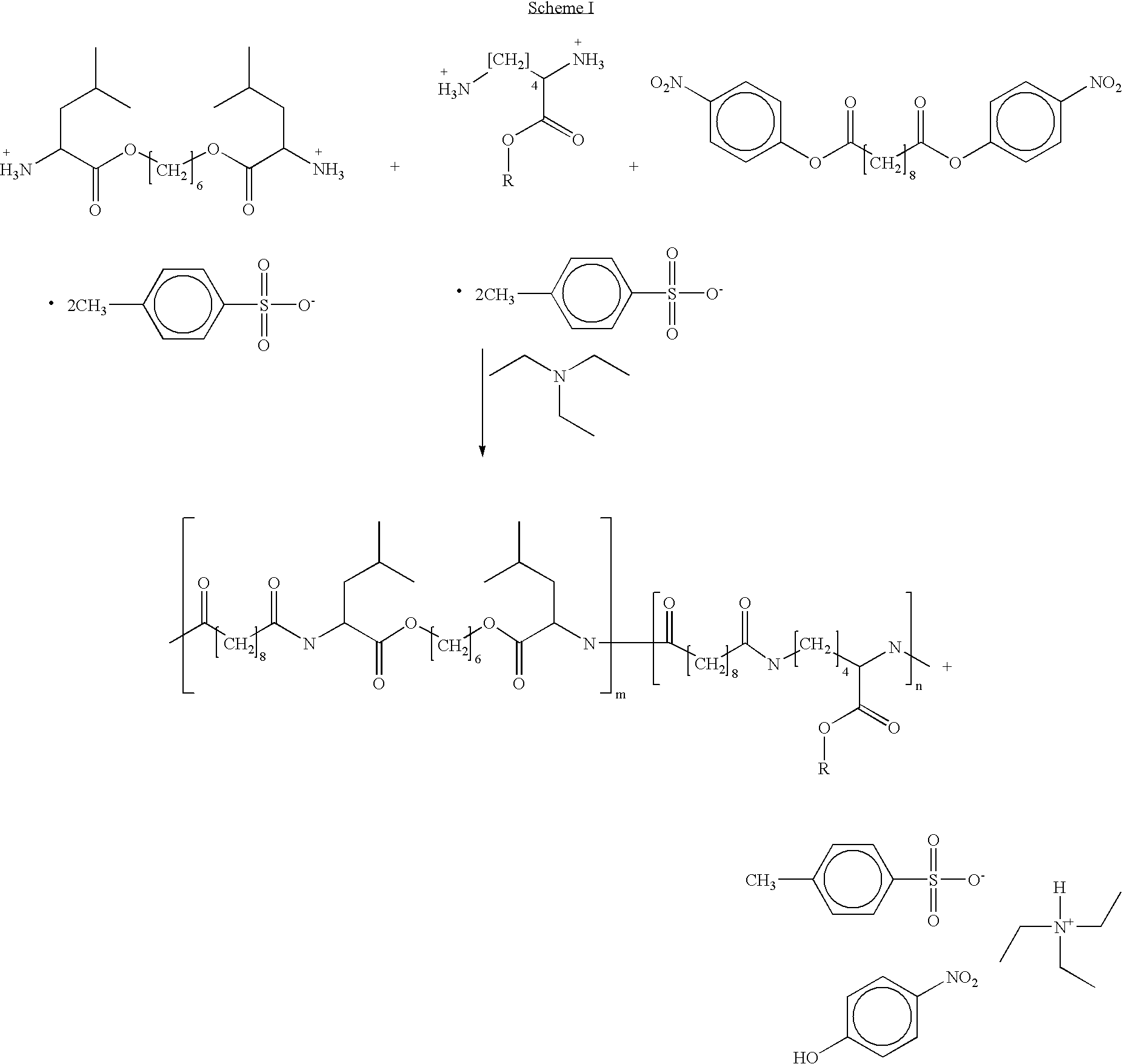
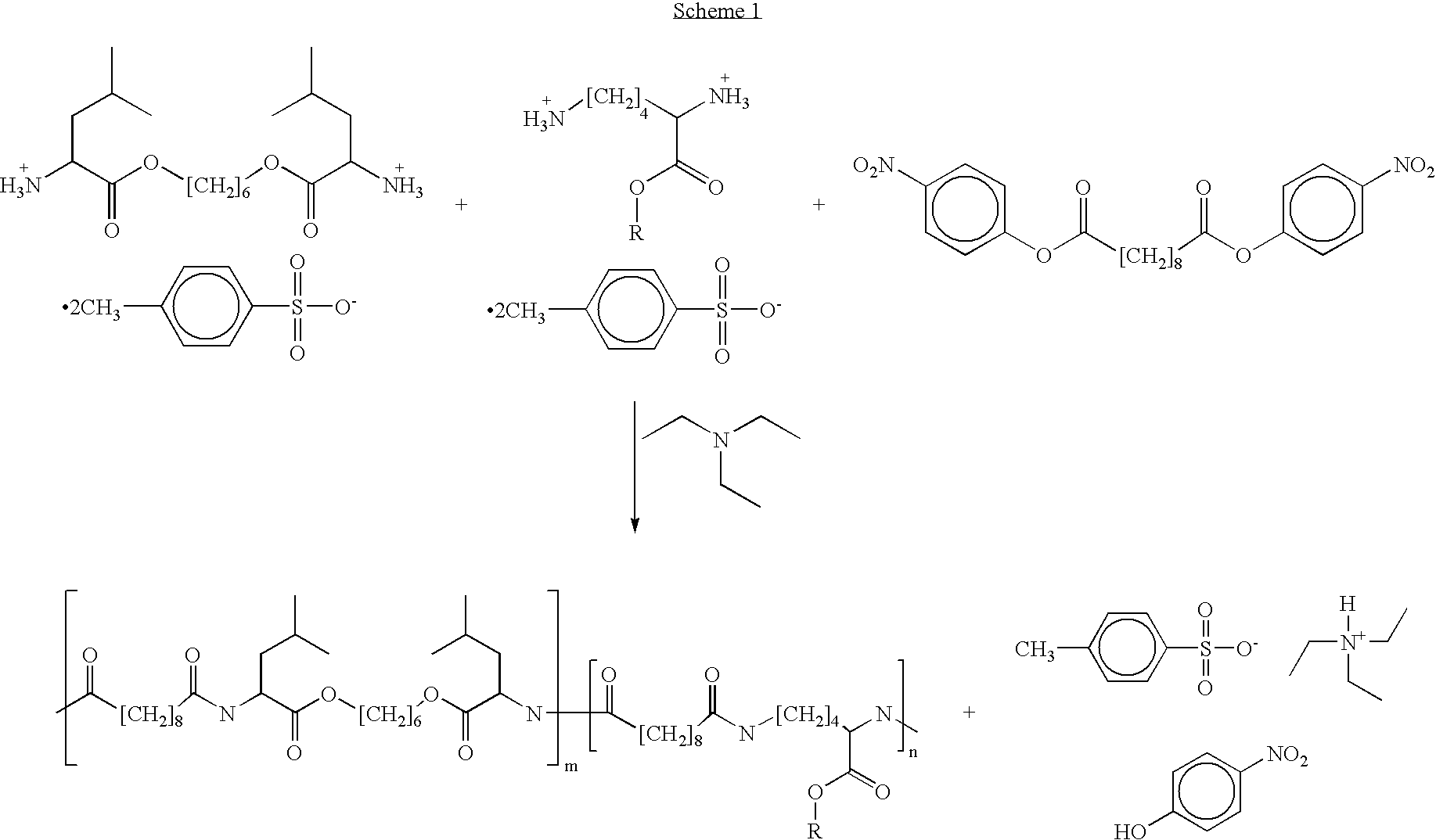
Blends of poly(ester amide) polymers
ActiveUS7166680B2Improved stability and drug release rate and mechanical characteristicReduce degradationSurgeryCatheterDiseasePEA polymer
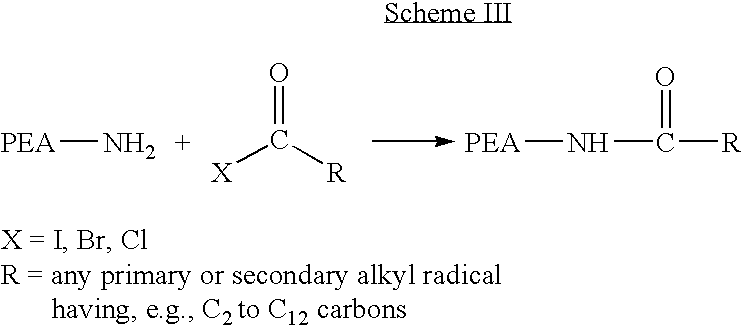
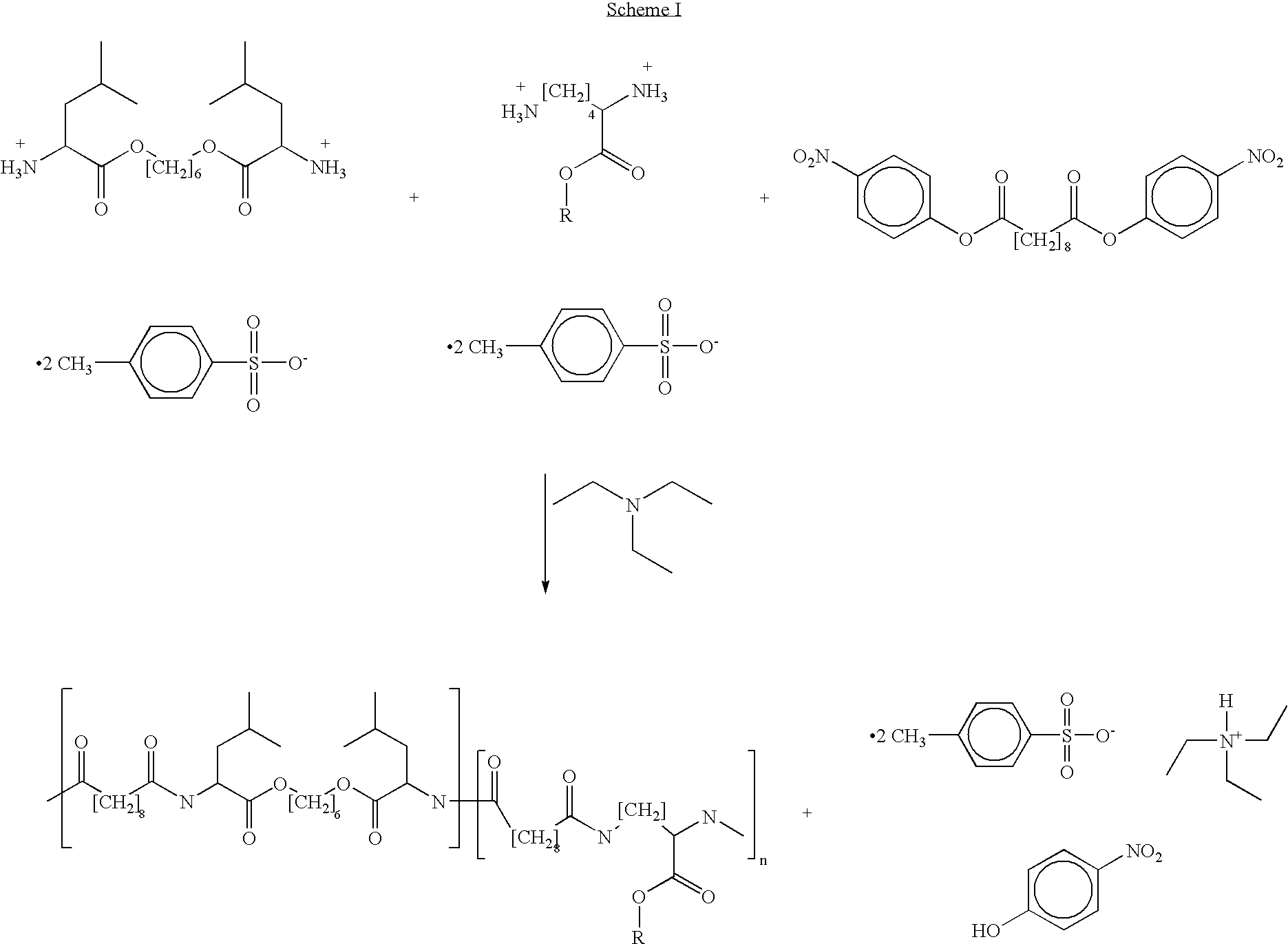
Provided herein is a poly(ester amide) (PEA) polymer blend and a polymeric coating containing the PEA polymer blend. The PEA polymer blend has a Tg above the Tg of poly(ester amide benzyl ester) (PEA-Bz) or the Tg of poly(ester amide TEMPO). The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Method and apparatus for treating wound using negative pressure therapy
InactiveUS7896823B2Easy to returnReduce edemaBlood stagnation preventionPneumatic massageVacuum assistedThrombus
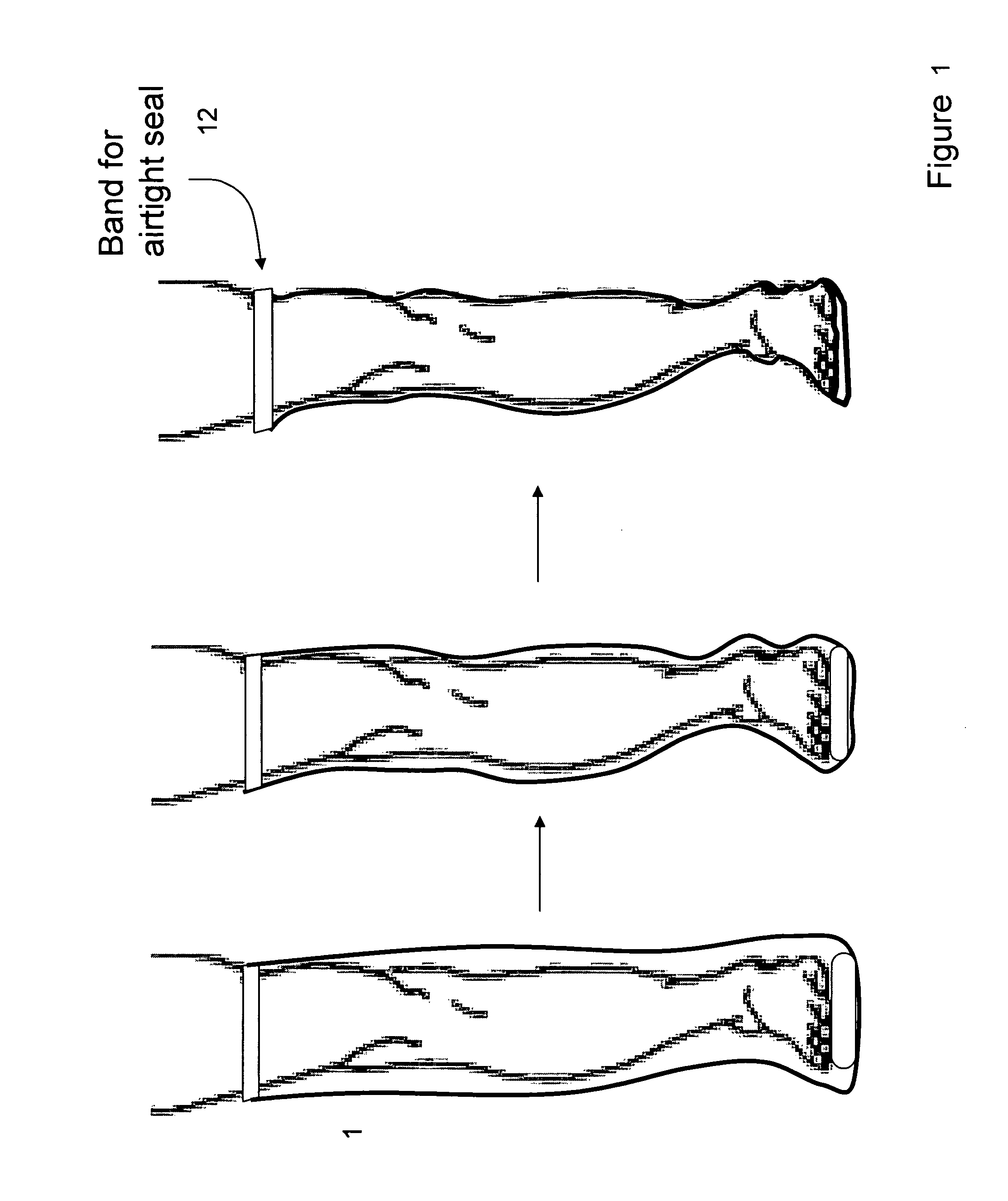
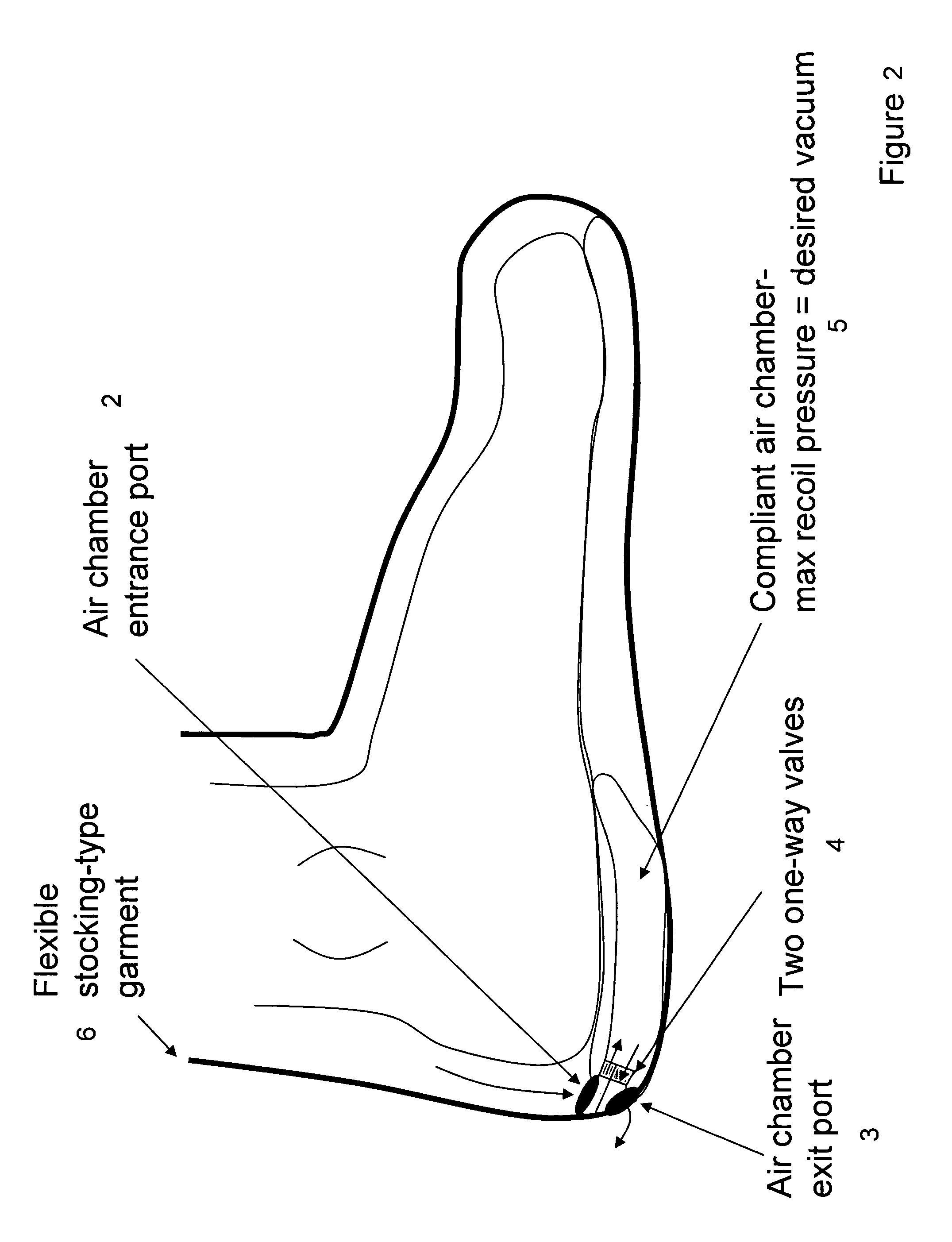
Devices that employ external compression stocking-type garments in the treatment of edema, chronic wounds, deep venous thrombosis prevention or claudication all share a number of significant limitations. These include the frequent need for custom fitting to assure an appropriate fit, vigilant maintenance to assure a continued “good fit,” limited compliance with proper use by patients and difficulty of application. There is a large body of evidence demonstrating that patients often decline to wear the compressive stockings as prescribed or in the form that would be most beneficial because they find these devices to be difficult to put on and take off.Building on the limitations of existing therapies, and distilled lessons learned from the field of prosthetics and wound healing, the present invention employs vacuum-assisted negative pressure to provide compression and help pump fluid from the tissues of affected limbs. The device is embodied in the form of a flexible stocking-like garment that will utilize a pumping mechanism to generate negative pressure around the limb and thus create vacuum compression that will mobilize fluid in a limb and increase venous return to the heart. Through the use of a circumferential wrap, the present invention provides a major advance in both the distribution of vacuum and the securing of the device over the limb.
Owner:THERANOVA LLC
End-capped poly(ester amide) copolymers
Provided herein is an end-capped poly(ester amide) PEA) polymer and the method of making the polymer. The PEA polymer is substantially free of active amino end groups and / or activated carboxyl groups. The PEA polymer can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Blends of poly(ester amide) polymers
Provided herein is a poly(ester amide) (PEA) polymer blend and a polymeric coating containing the PEA polymer blend. The PEA polymer blend has a Tg above the Tg of poly(ester amide benzyl ester) (PEA-Bz) or the Tg of poly(ester amide TEMPO). The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Poly(ester amide) filler blends for modulation of coating properties
InactiveUS20060093842A1Improve stabilityIncrease drug release rateOrganic active ingredientsNervous disorderAbnormal tissue growthPEA polymer
Provided herein is a PEA polymer blend and coatings or implantable devices formed therefrom. The PEA polymer blend is formed of a PEA polymer and a material capable of hydrogen bonding with the PEA. The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
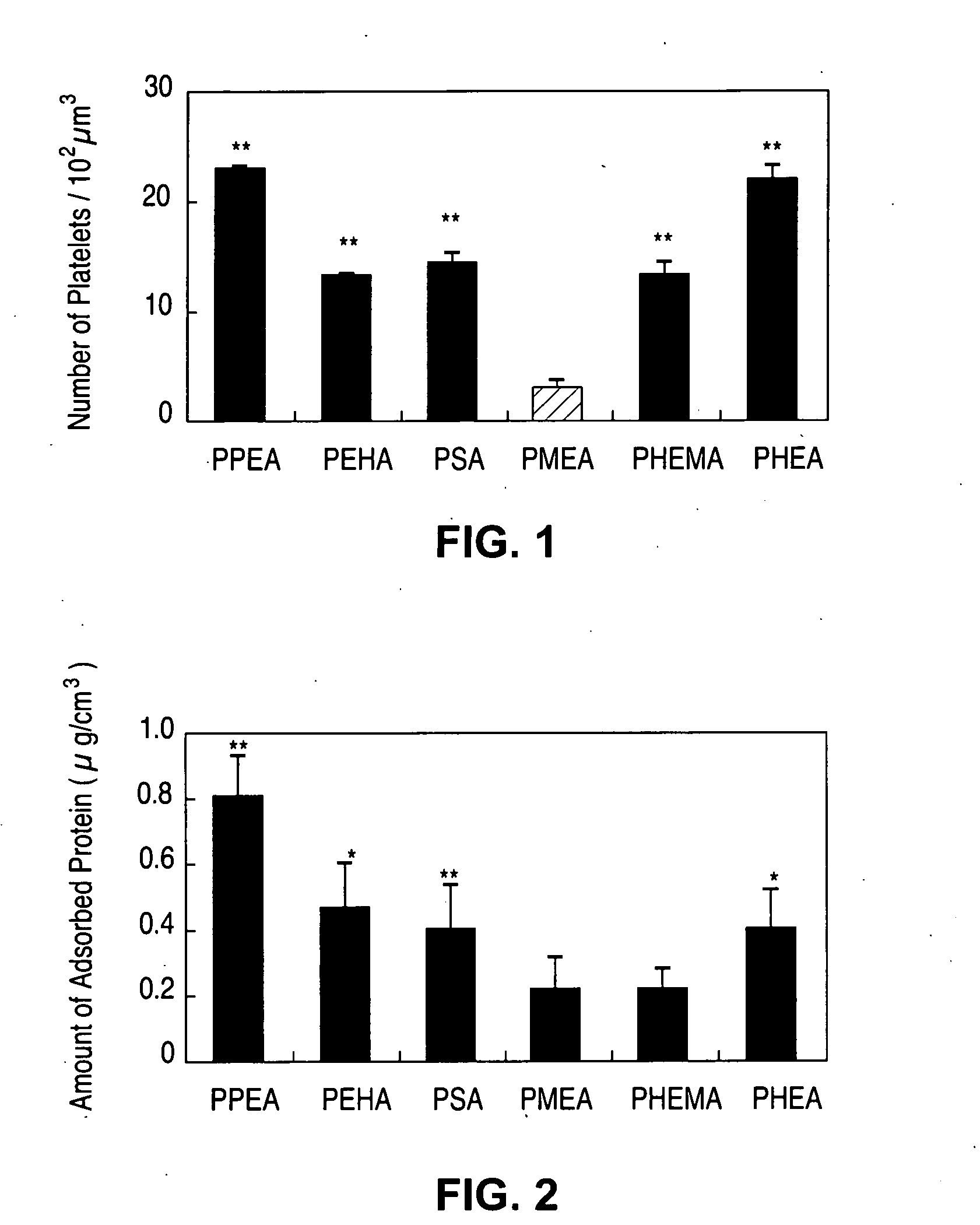
Implantable devices formed of non-fouling methacrylate or acrylate polymers
Implantable devices formed of or coated with a material that includes a polymer having a non-fouling acrylate or methacrylate polymer are provided. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
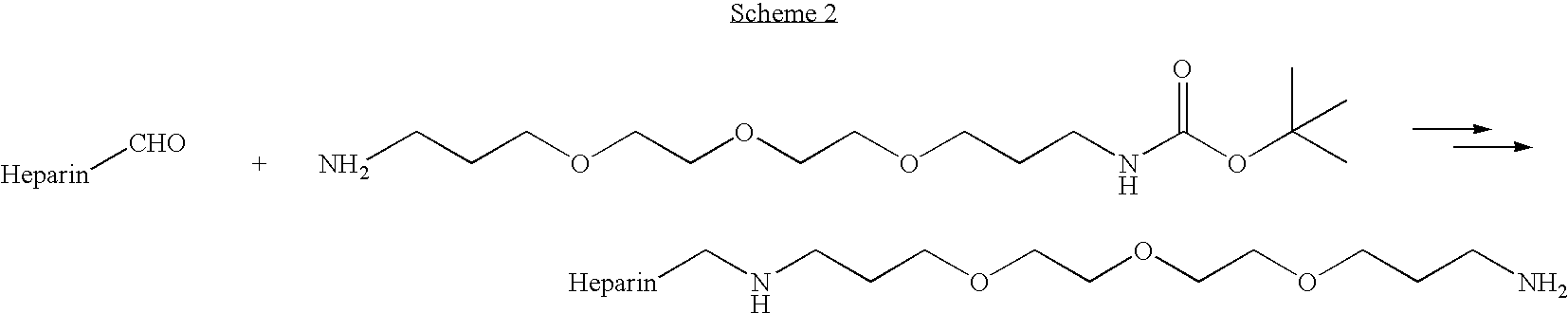
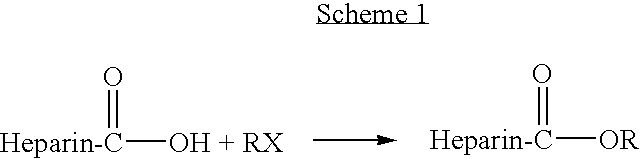
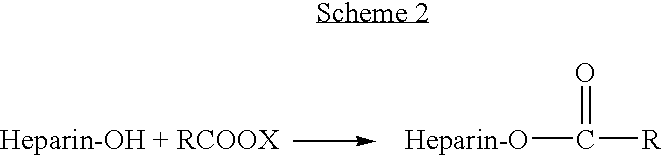
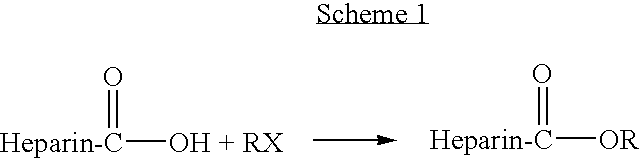
Heparin prodrugs and drug delivery stents formed therefrom
InactiveUS20060014720A1Organic active ingredientsPeptide/protein ingredientsDiseasePercent Diameter Stenosis
A prodrug comprising a heparin and a drug is provided. The prodrug can be used to form a coating on a medical device. The prodrug can also be used with a polymeric material to form a coating on a medical device. The polymeric material can be a hydrophobic polymer, a hydrophilic polymer, a non-fouling polymer, or combinations thereof. The medical device can be implanted in a human being for the treatment of a disease such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Poly(ester amide) filler blends for modulation of coating properties
InactiveUS7390497B2Improved stability and drug release rate and mechanical characteristicOrganic active ingredientsNervous disorderPEA polymerDevice form
Provided herein is a PEA polymer blend and coatings or implantable devices formed therefrom. The PEA polymer blend is formed of a PEA polymer and a material capable of hydrogen bonding with the PEA. The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Poly(ester amide) block copolymers
Owner:ABBOTT CARDIOVASCULAR
Beneficial effects of increasing local blood flow
ActiveUS20130289059A1Increase oxygenationImprove tissue nutritionBiocidePeptide/protein ingredientsArginineInjury mouth
The present invention generally relates to the improvement of tissue health by increasing local blood flow. In some aspects of the invention, increased local blood flow is effected by the transdermal delivery of the nitric oxide precursor L-arginine and / or its derivatives alone, or optionally in conjunction with an adjunct such as theophylline. The transdermal delivery is effected, in certain embodiments through the means of a hostile biophysical environment, such as that created by a high ionic strength environment. Various pathological states caused by, or occurring in conjunction with, insufficient blood flow, can be treated using the systems and methods of the invention as described herein. In other embodiments, increased blood flow using the systems and methods of the invention may result in enhanced healing, for example, through greater availability of the constituents of the blood. Examples of conditions which may benefit from increased blood flow include, but are not limited to, erectile dysfunction, hair loss, female sexual dissatisfaction, sagging facial or other body tissue, peripheral vascular disease including claudication, neuropathy, skin ulcers, bone healing, wound healing, viral and bacterial infection, and skin grafting.
Owner:STRATEGIC SCI & TECH
Heparin prodrugs and drug delivery stents formed therefrom
A prodrug comprising a heparin and a drug is provided. The prodrug can be used to form a coating on a medical device. The prodrug can also be used with a polymeric material to form a coating on a medical device. The polymeric material can be a hydrophobic polymer, a hydrophilic polymer, a non-fouling polymer, or combinations thereof. The medical device can be implanted in a human being for the treatment of a disease such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Implantable devices formed of non-fouling methacrylate or acrylate polymers
Implantable devices formed of or coated with a material that includes a polymer having a non-fouling acrylate or methacrylate polymer are provided. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Polymers containing siloxane monomers
Owner:ABBOTT CARDIOVASCULAR
Beneficial effects of increasing local blood flow
ActiveUS9226909B2Increase ionic strengthReduce deliveryBiocidePeptide/protein ingredientsArginineInjury mouth
The present invention generally relates to the improvement of tissue health by increasing local blood flow. In some aspects of the invention, increased local blood flow is effected by the transdermal delivery of the nitric oxide precursor L-arginine and / or its derivatives alone, or optionally in conjunction with an adjunct such as theophylline. The transdermal delivery is effected, in certain embodiments through the means of a hostile biophysical environment, such as that created by a high ionic strength environment. Various pathological states caused by, or occurring in conjunction with, insufficient blood flow, can be treated using the systems and methods of the invention as described herein. In other embodiments, increased blood flow using the systems and methods of the invention may result in enhanced healing, for example, through greater availability of the constituents of the blood. Examples of conditions which may benefit from increased blood flow include, but are not limited to, erectile dysfunction, hair loss, female sexual dissatisfaction, sagging facial or other body tissue, peripheral vascular disease including claudication, neuropathy, skin ulcers, bone healing, wound healing, viral and bacterial infection, and skin grafting.
Owner:STRATEGIC SCI & TECH
Antifouling heparin coatings
Owner:ABBOTT CARDIOVASCULAR
Polymers of fluorinated monomers and hydrocarbon monomers
InactiveUS7604818B2Provide mechanical strengthGive flexibilityStentsFibre treatmentPolymer scienceActive agent
A polymer of fluorinated monomers and hydrocarbon monomers is provided. It is also provided a polymer blend that contains a polymer formed of fluorinated monomers and hydrocarbon monomers and another biocompatible polymer. The polymer or polymer blend described herein and optionally a bioactive agent can form an implantable device such as a stent or a coating on an implantable device such as a drug-delivery stent, which can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Methacrylate copolymers for medical devices
A polymer of hydrophobic monomers and hydrophilic monomers is provided. It is also provided a polymer blend that contains the polymer and another biocompatible polymer. The polymer or polymer blend and optionally a biobeneficial material and / or a bioactive agent can form a coating on an implantable device such as a drug delivery stent. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
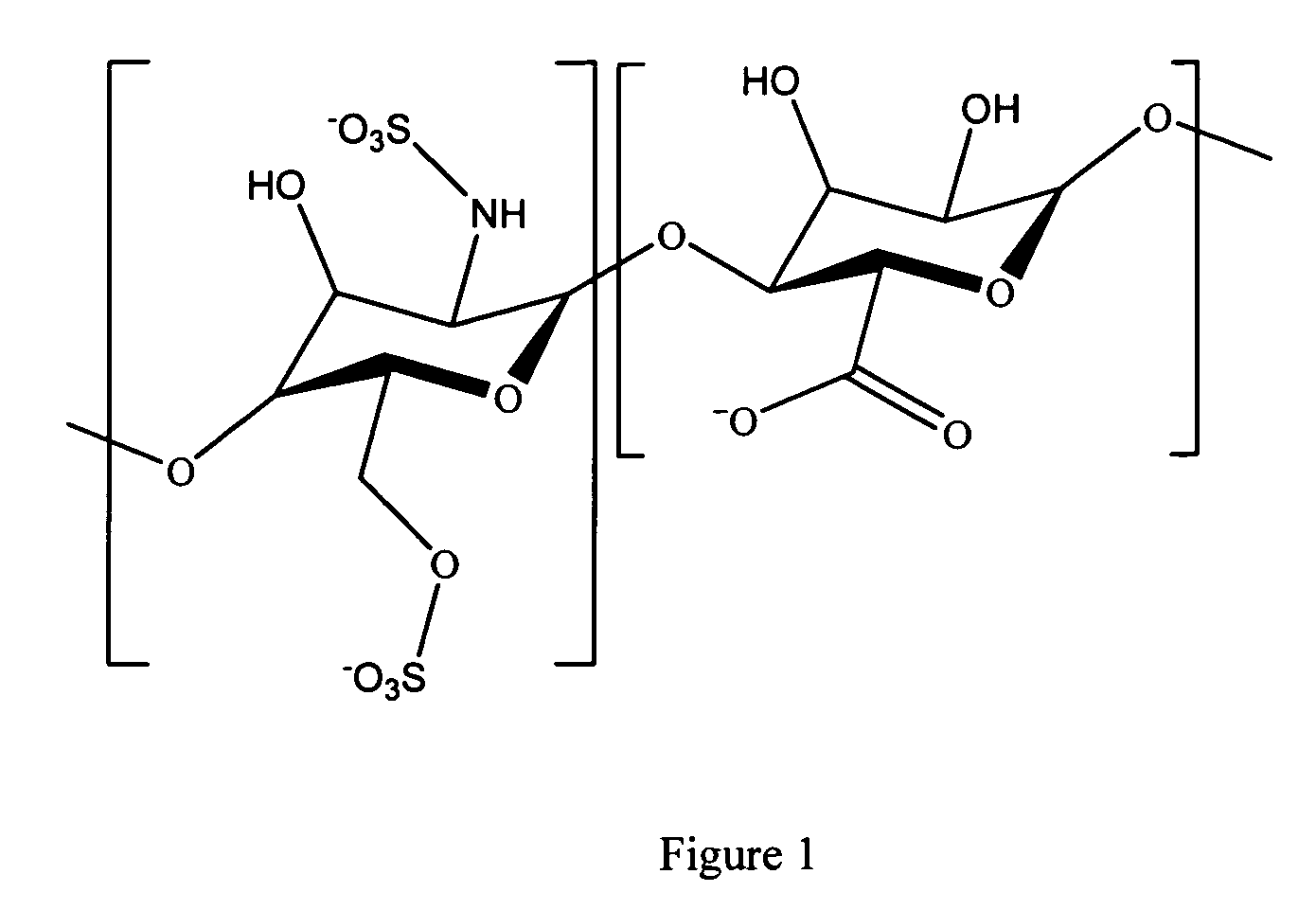
Hyaluronic acid based copolymers
Owner:ABBOTT CARDIOVASCULAR
Chinese medicine composition orally taken for curing cervical vertebra osteoproliferation
InactiveCN101181597AAnthropod material medical ingredientsSkeletal disorderOsteoproliferationHeadaches
The invention discloses a Chinese traditional medicine combination which is internally used for curing cervical vertebra hyperosteogeny, belonging to the field of traditional Chinese medicine. The invention is characterized in that the active ingredients of the traditional Chinese medicine combination are prepared by the following raw materials by weight: 15g prepared rhizome of rehmannia, 12g of dogwood, 15g of medlars, 10g of dodder, 30g of radix salviae miltiorrhizae, 21g of leatherleaf milletia, 12g of rhizoma corydalis, 21g of garden balsam stems, 12g curcuma, 10g of pangolin and 12g of earthworm. When preparing the medicine, the impurities in the raw materials are removed, and the materials are decocted with water, then the medicine is taken one dose and twice per day, morning and night. The invention can achieve the effects for dissipating blood stasis, removing obstruction in collaterals, relieving pain and eliminating swelling, fundamentally restraining hyperosteogeny, effectively promoting blood circulation, and repairing local damage of tissue, thereby achieving the aim of eliminating a series of symptoms such as dizziness, headache, myasthenia of limbs, interim claudication, arms and shoulders acroanesthesia, pain in waist, legs, feet and heel, and the symptom that the patients can not take care of themselves caused by dedifferentiation such as hyperosteogeny, etc.
Owner:胡玉翠
Drug for treating arteriosclerosis
InactiveCN102671128AEasy to manufactureQuick resultsAnthropod material medical ingredientsCardiovascular disorderHeavy smokingMyrrh
The invention provides a drug for treating arteriosclerosis, which is prepared by the following traditional Chinese medicines of: white peony, hawthorn, cherokee rose fruit, Codonopsis pilosula, lophatherum gracile, rhizoma atractylodis, silkworm larva, Polygonum cuspidatum, semen cassia, rhizoma alismatis, radix bupleuri, radix curcumae, fruit of Chinese wolfberry, Poria cocos, plantain seed, white atractylodes rhizome, Manis pentadactyla, gastrodia elata, Ligusticum wallichii, trigone, zedoary, leech, Polygonum multiflorum, frankincense, myrrh and oyster. The drug provided by the invention has better treatment effects on the common symptoms of the patients with arteriosclerosis, such as palpitation, flustering, chest pain, choking sensation in chest, headache, dizziness, cold and numb extremities, soreness and weakness of limbs, claudication, blurred vision, poor memory, insomnia and dreaminess, and also has better preventive effect on the city dwellers, the person who engages in intense brain works, the obese person, the heavy smoking person, the patient with hypertension, the patient with diabetes mellitus, the patient with hyperlipemia and the population susceptible to the arteriosclerosis; and the drug has the outstanding effects of no toxic or side effects, no untoward effect, and high cure rate.
Owner:王静 +1
Method of treatment of connective tissue disorders by administration of streptolysin O
ActiveUS6998121B2Relieve symptomsEffective treatmentNervous disorderPeptide/protein ingredientsSpinal claudicationConnective tissue
Method for administering streptolysin O to treat various connective tissue disorders in humans and animals such as Dupuytren's contracture, scleroderma, Peyronie's disease, mastistis in animals, and claudication due to peripheral arterial disease.
Owner:MILKHAUS LAB
Methods and compositions for the treatment of peripheral artery disease
InactiveUS7186407B2Inhibit progressEffective treatmentOrganic active ingredientsNervous disorderSpinal claudicationVein
Compositions and methods for treating peripheral artery disease in a patient are provided. Compositions comprise recombinant fibroblast growth factor-2. Fibroblast growth factor, such as FGF-2, is administered in therapeutically effective amounts to treat or prevent peripheral artery disease including claudication and critical limb ischemia. Pharmaceutical compositions comprising a therapeutically effective amount of FGF-2 and a pharmaceutically acceptable carrier are also provided. The methods of the invention to treat peripheral artery disease and claudication comprise administering at least a single dose of a pharmaceutical composition comprising the FGF, such as FGF-2, via intra-arterial, intravenous, or intramuscular infusion to the patient. It is recognized that increased benefits may result from multiple dosing, including intermittent dosing.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Traditional Chinese medicine preparation for treating arteriosclerosis
InactiveCN103610916AGood treatment effectDefinite curative effectCardiovascular disorderPlant ingredientsSide effectPalpitations
The invention discloses a traditional Chinese medicine preparation for treating arteriosclerosis and belongs to the field of traditional Chinese medicines. The active ingredients of the traditional Chinese medicine preparation comprise the following raw materials: sophora flower bud, hawthorn, radix puerariae, walnut kernel, radix polygonati officinalis, watermeion peel, uncaria, rhizoma alismatis, lucid ganoderma, rehmannia glutinosa, three-colored amaranth, fig, polygonum cuspidatum, pollen typhae, parasitic loranthus and pumpkin. The traditional Chinese medicine preparation has the effects of reinforcing qi and nourishing blood, promoting blood circulation to remove blood stasis and softening blood vessels, has an obvious effect of treating common symptoms such as palpitation, chest pain, choking sensation in chest, headache, dizziness, coldness and anaesthesia of the four limbs, aching and tired limbs, claudication, vision decrease, memory deterioration, insomnia and dreaminess occurring in arteriosclerosis patients, is accurate in curative effect and low in cost, does not have any toxic or side effect and is worthy of wide clinical popularization and application.
Owner:董金永
Implantable devices formed on non-fouling methacrylate or acrylate polymers
Implantable devices formed of or coated with a material that includes a polymer having a non-fouling acrylate or methacrylate polymer are provided. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com