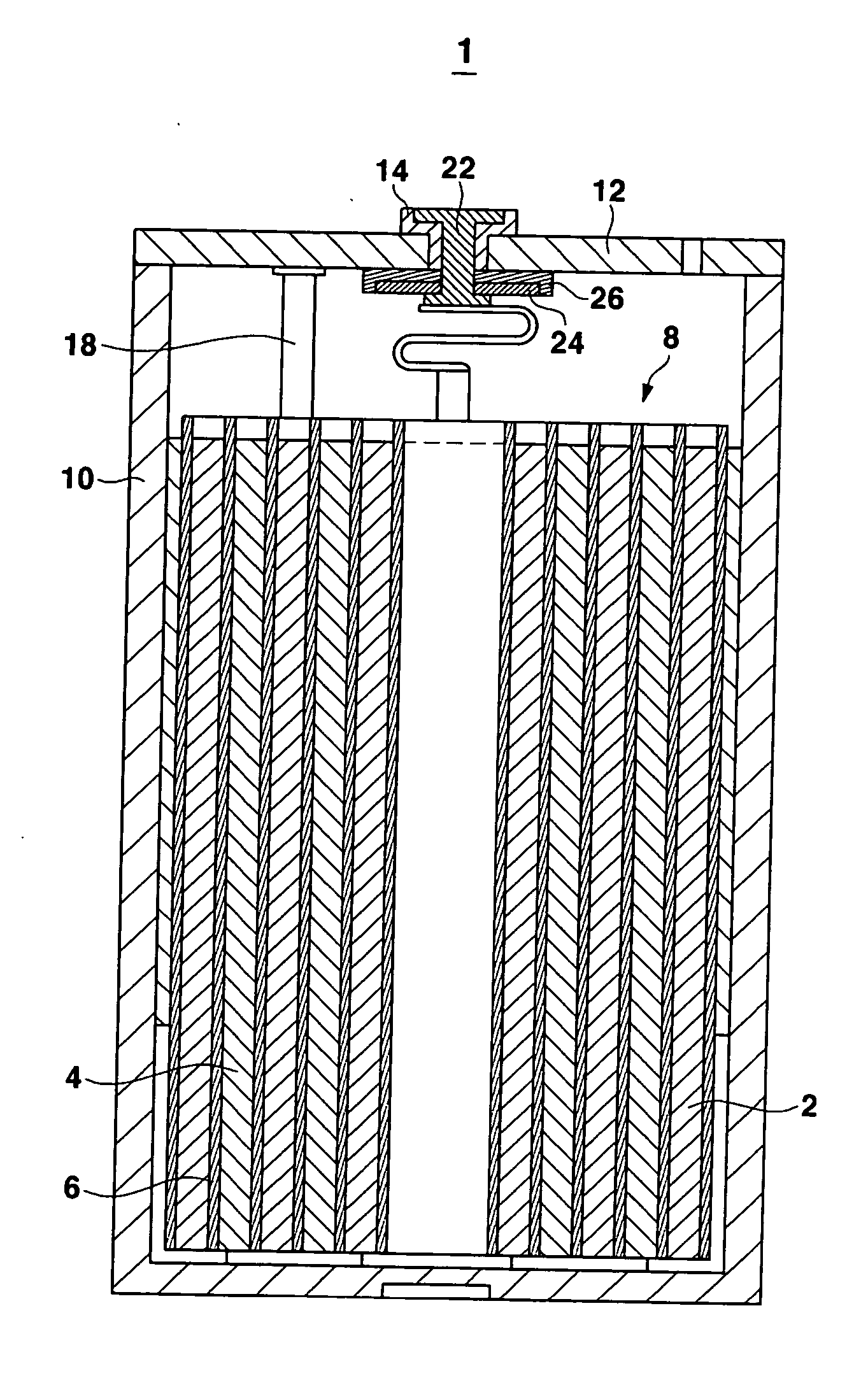
Electrolyte for a lithium battery and a lithium battery comprising the same
a lithium battery and electrolyte technology, applied in the direction of non-aqueous electrolyte cells, furniture parts, electrochemical generators, etc., can solve the problems of increasing the need for high-capacity batteries, seriously reducing battery safety, and thermal instability of positive and negative electrodes, so as to improve battery safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experimental example 1
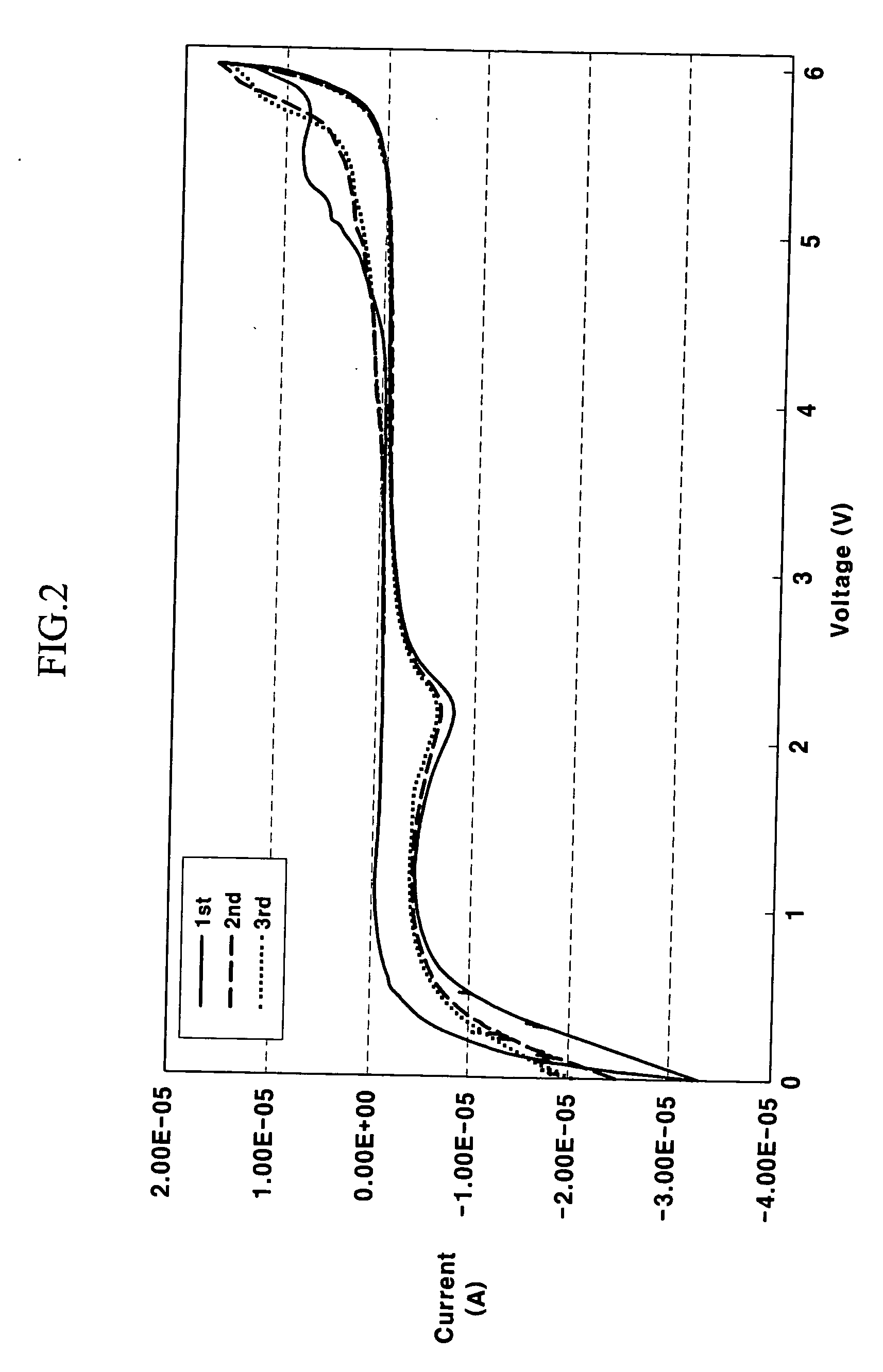
[0053] A working electrode was prepared using glassy carbon, and a reference electrode and counter electrode was prepared using lithium metal. Cyclic voltammetry of succino nitrile was then measured three times at a scanning rate of 0.5 mV / second. The results are shown in FIG. 2. As shown in FIG. 2, succino nitrile did not show an oxidation-reduction peak between 2.5 and 4.8 V, indicating that this compound is stable in this voltage range.
experimental example 2
[0054] A positive electrode was prepared as in Comparative Example 1 and subjected to the standard charge conditions. The positive electrode was then dipped in an electrolyte solution. Subsequently, phenyl acetate and a first additive capable of forming a chelating complex were added to the positive electrode, and the positive electrode was then stored at 85° C. for four hours. Table 2 lists the first additive used and the color of the electrolyte solution.
TABLE 2Color after storage atInitial color85° C. for 4 hoursExample 1TransparentDeep orangeSuccino nitrileTransparentpale yellowAceto nitrileTransparentLight pinkValero nitrileTransparentLight pink3-ethoxy-propionitrileTransparentLight pinkEthylene glycol diacrylateTransparentPink1,2-Pale yellowYellowbis(diphenylphosphino)ethane1,2-dibromoethaneTransparentPale orangeEthylenediamineYellowDeep brownTetraethylenediamineYellowDeep brown
[0055] Table 2 shows that after cobalt was released a complex was formed resulting in a change of ...
example 1
[0056] A lithium secondary battery was fabricated as in Comparative Example 1, except that the electrolyte was prepared by adding succino nitrile to a solution of 1 M LiPF6 dissolved in a mixed solvent of ethylene carbonate, ethylmethyl carbonate, dimethyl carbonate and fluorobenzene. The volume ratio of ethylene carbonate: ethylmethyl carbonate:dimethyl carbonate:fluorobenzene was 3:5:5:1. The succino nitrile was added in an amount of 5 wt % based on the total weight of electrolyte.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com