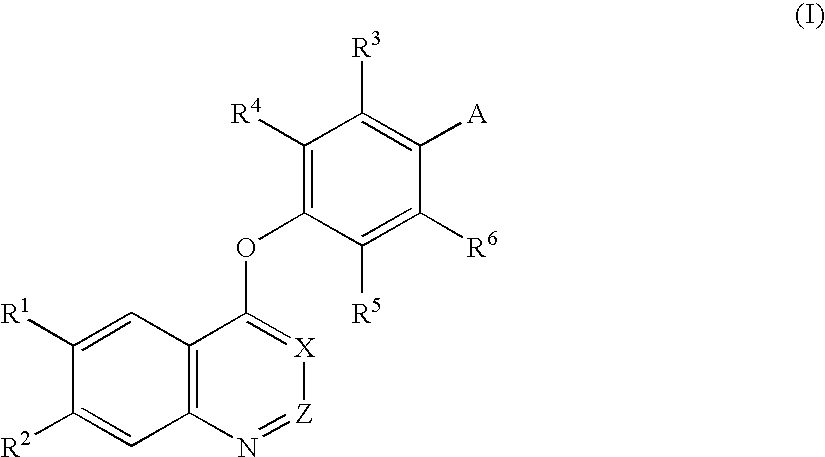
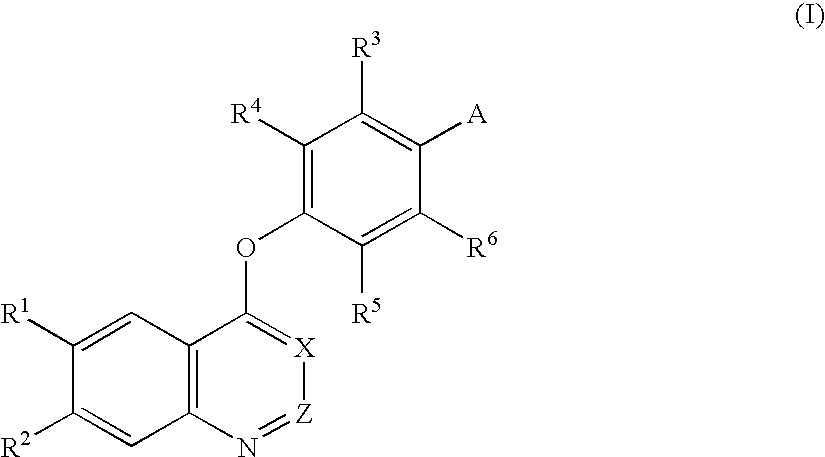
Quinoline and quinazoline derivatives and drugs containing the same
a technology of quinazoline and quinoline, which is applied in the direction of biocide, cardiovascular disorder, drug composition, etc., can solve the problems of hematopoietic failure and intestinal tract movement failure, pharmaceutical compounds with potent angiostenosis inhibitory activity that have not yet been developed, and achieve low side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
production example 1
4-[(6,7-Dimethoxy-4-quinazolinyl)oxy]-2-nitroaniline
[0220] Sodium hydride (60 wt %, 0.20 g) was added to dimethyl sulfoxide (15 ml), and the mixture was stirred at room temperature for 10 min. 4-Amino-3-nitrophenol (0.77 g) was added thereto, and the mixture was stirred at room temperature for 10 min. Next, 4-chloro-6,7-dimethoxyquinazoline (1.12 g) was added thereto, and the mixture was stirred at 100° C. for 3 hr. Water was added to the reaction solution, followed by extraction with chloroform. The chloroform layer was then washed with a 1 N aqueous sodium hydroxide solution and was dried over anhydrous sodium sulfate. The solvent was removed by distillation under the reduced pressure, and methanol was added to the residue to prepare a suspension. The precipitated crystal was collected by suction filtration to give the title compound (1.10 g, yield 64%).
[0221]1H-NMR (CDCl3-d1, 400 MHz): δ 4.07 (s, 3H), 4.08 (s, 3H), 6.10-6.15 (m, 2H), 6.92 (d, J=9.0 Hz, 1H), 7.34 (s, 1H), 7.35 (...
production example 2
4-[(6,7-Dimethoxy-4-quinolyl)oxy]-3-fluoroaniline
[0223] 4-Chloro-6,7-dimethoxyquinazoline (10.23 g) and 2-fluoro-4-nitrophenol (14.37 g) were suspended in monochlorobenzene (100 ml), and the suspension was heated under reflux overnight. The solvent was removed by distillation under the reduced pressure, and the residue was washed with toluene, was filtered, and was dried. The crystal thus obtained was then suspended in an aqueous sodium hydroxide solution, and the suspension was filtered, followed by drying to give 4-(3-fluoro-4-nitrophenoxy)-6,7-dimethoxyquinoline (14.2 g, yield 90%). 4-(2-Fluoro-4-nitrophenoxy)-6,7-dimethoxy-quinoline (4.57 g) was dissolved in ethyl acetate / N,N-dimethylformamide / triethylamine (100 ml / 100 ml / 20 ml) to prepare a solution. Palladium hydroxide (1.2 g) was added to the solution, and the mixture was stirred in a hydrogen atmosphere at room temperature overnight. After filtration through Celite, the solvent was removed by distillation under the reduced ...
production example 3
3-Chloro-4-[(6,7-dimethoxy-4-quinolyl)oxy]aniline
[0226] Sodium hydride (60 wt %, 0.72 g) was added to dimethyl sulfoxide (10 ml), and the mixture was stirred at 50° C. for 20 min. 4-Amino-3-chlorophenol hydrochloride (1.61 g) was added thereto, and the mixture was stirred at room temperature for 10 min. Next, 4-chloro-6,7-dimethoxyquinoline (1.00 g) was added thereto, and the mixture was stirred at 100° C. overnight. Water was added to the reaction solution, and the mixture was extracted with chloroform. The chloroform layer was then washed with a saturated aqueous sodium hydrogencarbonate solution and was dried over anhydrous sodium sulfate. The solvent was removed by distillation under the reduced pressure. Methanol was added to the residue, and the precipitated crystal was collected by suction filtration to give the title compound (0.80 g, yield 60%).
[0227]1H-NMR (CDCl3-d1, 400 MHz): δ 4.06 (s, 3H), 4.07 (s, 3H), 6.36 (d, J=5.4 Hz, 1H), 6.65 (dd, J=8.5 Hz, J=2.9 Hz, 1H), 6.76 (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com