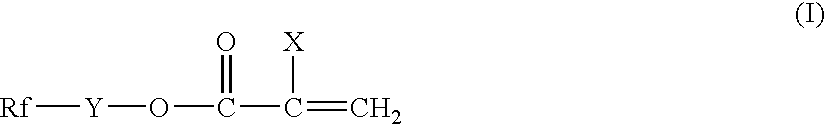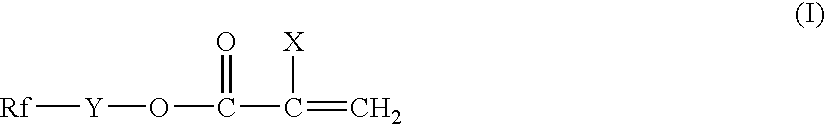Masonry-treating agent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0079] Into a 200 cc four-necked flask equipped with a stirrer, an inert gas inlet, a condenser and a thermometer, 13.0 g of CF3CF2CF2CF2CH2CH2OCOCCl═CH2, 6.5 g of stearyl acrylate, 0.5 g of γ-methacryloxypropyltrimethoxysilane (SZ6030 manufactured by Dow Corning Toray Co., Ltd.) and 113.3 g of tetrachlorohexafluorobutane (S-316 manufactured by Daikin Industries, Ltd.) were charged and heated to 60° C. A solution of t-butyl peroxypivalate (1.5 g) (PERBUTYL PV manufactured by NOF Corp.) in trichloroethane (7.3 g) was added and the polymerization reaction was conducted with stirring at 60° C. for at least 12 hours. A gas chromatography revealed that a polymerization reaction conversion was at least 97%. The resultant polymer solution was diluted with butyl acetate to give a treatment liquid having a solid content of 3%.
[0080] A surface of each of polished natural granite (mined in China, and purchased from Nittai Kogyo Kabushiki-Kaisha) and limestone (purchased from Inax Corp.) was c...
example 2
[0083] Into a 200 cc four-necked flask equipped with a stirrer, an inert gas inlet, a condenser and a thermometer, 16.8 g of CF3CF2CF2CF2CH2CH2OCOCCl═CH2, 2.7 g of stearyl acrylate, 0.5 g of γ-methacryloxypropyltrimethoxysilane (SZ6030 manufactured by Dow Corning Toray Co., Ltd.) and 60 g of butyl acetate were charged and heated to 70° C. Azobisisobutyronitrile (0.15 g) was added and the polymerization reaction was conducted with stirring at 70° C. for at least 12 hours. A gas chromatography revealed that a polymerization reaction conversion was at least 97%. In the same manner as in Example 1, the polymer solution was diluted with butyl acetate to adjust the solid concentration to 3%, the treatment was conducted and the soil resistance test was conducted. The results are shown in Table 1 and Table 2.
example 3
[0084] The polymerization reaction was conducted to give a polymer solution in the same procedure as in Example 1 except that CF3CF2CF2CF2CH2CH2OCOCCl═CH2 was changed to CF3CF2CF2CF2CH2CH2OCOCF═CH2. In the same manner as in Example 1, the polymer solution was diluted with butyl acetate to adjust the solid concentration to 3%, the treatment was conducted and the soil resistance test was conducted. The results are shown in Table 1 and Table 2.
TABLE 1Soil resistance test (granite)Olive OilWaste oilRed wineCoffeeExample 15545Comparative Example 12222Comparative Example 23323Untreated1111Example 25555Example 35445
[0085]
TABLE 2Soil resistance test (limestone)Olive OilWaste oilRed wineCoffeeExample 15445Comparative Example 12222Comparative Example 23223Untreated1111Example 25555Example 35445
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com