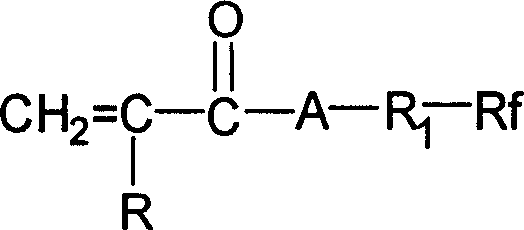
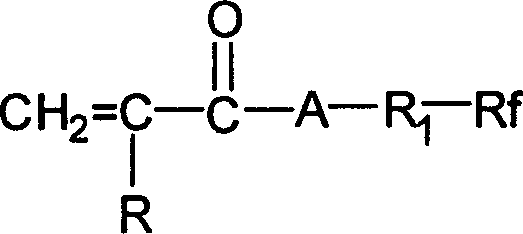
Shell-core type fluoride containing emulsion
A core-shell type latex technology, which is applied in the field of core-shell fluorine-containing acrylate latex, can solve the problems of restricting the wide application of fluorine-containing latex, high cost and high price of fluorine-containing latex, and achieve easy control, lower latex cost, The effect of reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Mix the following ingredients in a 1000ml beaker: 100g of butyl methacrylate, 2g of divinylbenzene, 3g of sodium lauryl sulfate-OP-10 composite emulsifier, 400 g of deionized water, stir at high speed to obtain a stable pre-emulsion, and obtain a stable The pre-emulsion I. Pre-emulsion I is added in the reactor that agitator, feeder, thermometer and condenser are equipped with, then add initiator sodium persulfate-ferrous salt 0.6g, sodium bicarbonate 0.8g, logical nitrogen deoxygenation, stirring and heating, The temperature of the water bath was raised to 50° C., and opalescence appeared after 3 minutes, and the seed emulsion was obtained. At the same time, place the remaining monomers, emulsifier, dodecanethiol, and 100g of water in an SK1200H ultrasonic cleaner for pre-emulsification for 0.5 hours, and obtain the shell pre-emulsion II through ultrasonic pre-emulsification, and then drop the shell pre-emulsion and the initiator solution at the same time into the see...
Embodiment 2
[0029] 100g of butyl acrylate, 2g of perfluoroalkylethyl methacrylate, 3g of octadecyltrimethylammonium chloride, and 300g of deionized water were placed in a USC-102 ultrasonic cleaner for pre-emulsification for 1 hour to obtain stable seed pre-emulsion. Add the seed pre-emulsion to the reaction kettle equipped with agitator, thermometer, feeder and reflux condenser, then add initiator ammonium persulfate-sodium bisulfite-copper sulfate 0.6g, sodium hydrogen phosphate 0.8g, and nitrogen to remove Oxygen, stirred and heated, the temperature of the water bath was raised to 40°C, opalescence appeared soon, and the seed emulsion was obtained. At the same time, the remaining monomers, emulsifier, mercaptoethanol, and 100 g of water were also ultrasonically pre-emulsified to obtain a shell pre-emulsion, and then the shell pre-emulsion and the initiator solution were added dropwise to the seed emulsion at the same time, and the reaction was continued for 5 hours to obtain a shell pr...
Embodiment 3~6
[0033] Except for some monomers, other conditions are the same as in Example 1 or 2, and the specific monomers and their dosage are shown in Table 3.
[0034] The monomer consumption (weight part) in the embodiment 3-6 of table 3
[0035] Monomer Example 3 Example 4 Example 5 Example 6
[0036] Lower (meth)acrylate 100 100 100 100
[0037] Divinylbenzene 2 0 - 0
[0038] Perfluoroalkylethyl (meth)acrylate 30 75 30 30
[0039] Advanced (meth)acrylate 10 20 - 8
[0040] Vinylidene chloride 3 - - 3
[0041]Hydroxyalkyl (meth)acrylate 1 - - 1
[0042] Diacrylate crosslinking monomer 1 1 - -
[0043] N-Hydroxyacrylamide 1 2 - 2
[0044] In Table 3: said (meth)acrylic acid lower ester contains C 1 ~C 8 (meth)acrylates;
[0045] The (meth)acrylic higher esters contain C 12 ~C 16 (meth)acrylates;
[0046] Said diacrylate crosslinking monomer contains C 2 ~C 12 Diacrylate crosslinking monomer;
[0047] Said perfluoroalkylethyl (meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com