Modified release formulations of antihypertensive drugs
a technology of antihypertensive drugs and modified release formulations, applied in the field of propranolol compound, can solve the problems of difficult control of the release of the difficulty of putting into the modified release formulation, and the difficulty of achieving the effect of reducing the risk of cardiovascular diseas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
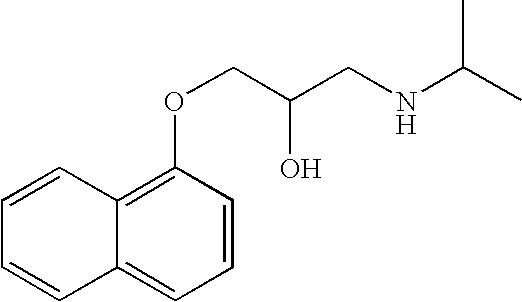
[0084] Propranolol HCl (160 mg) and Microcrystalline cellulose powder (101.82 mg) are mixed into a blend with a high shear granulator for 15 minutes. A clear binder solution of ethyl cellulose N100 (8.44 mg) in sufficient amount of Isopropyl alcohol is made. The blend is further granulated with slow addition of the binder solution for 45 min. The granules are dried in a Thelco lab dryer at about 50° C. for about 1 hour. A sufficient amount of water is then added to facilitate extrusion. The resulting mass is extruded through a 1 mm mesh and then spheronized in a spheronizer to create granules. The granules are again dried in a Thelco lab dryer at about 50° C. till the moisture content is less than about 1.0% and solvent content is less than about 0.1% to yield beads with average of 850 um. The beads are then film-coated with a solution of ethylcellulose N100 (14.55 mg) in isopropyl alcohol with triethyl citrate (1.46 mg) as plasticizer in a conventional coating pan. The ...
example 2
[0086] Propanolol containing cores are prepared as in Example No. 1. The cores containing 160 mg of propranolol hydrochloride are then coated with a solution of Methocel E5 LV premium (3.24 mg) and Ethylcellulose N100 (7.57 mg) in methanol with triethyl citrate (1.08 mg) as plasticizer coating layer using a fluid bed apparatus. A Glatt GPCG 3.1 can be used for this purpose. Fill the capsule size “1” with sufficient amount of beads so that the total Propranolol HCl content is 160 mg.
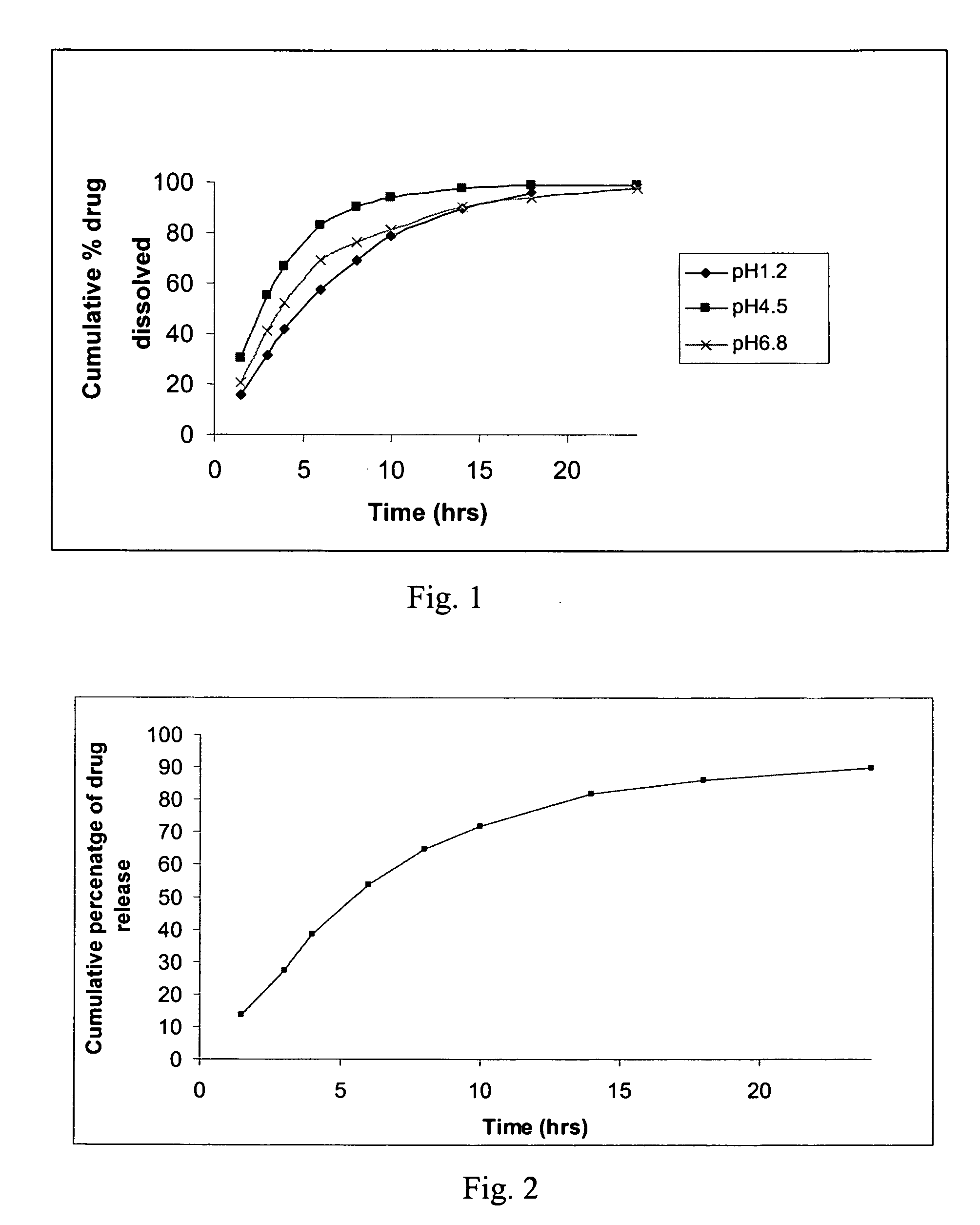
[0087] Dissolution was conducted according to the protocol set forth in Example 10. The results thereof are shown in graphical form in FIG. 2.
example 3
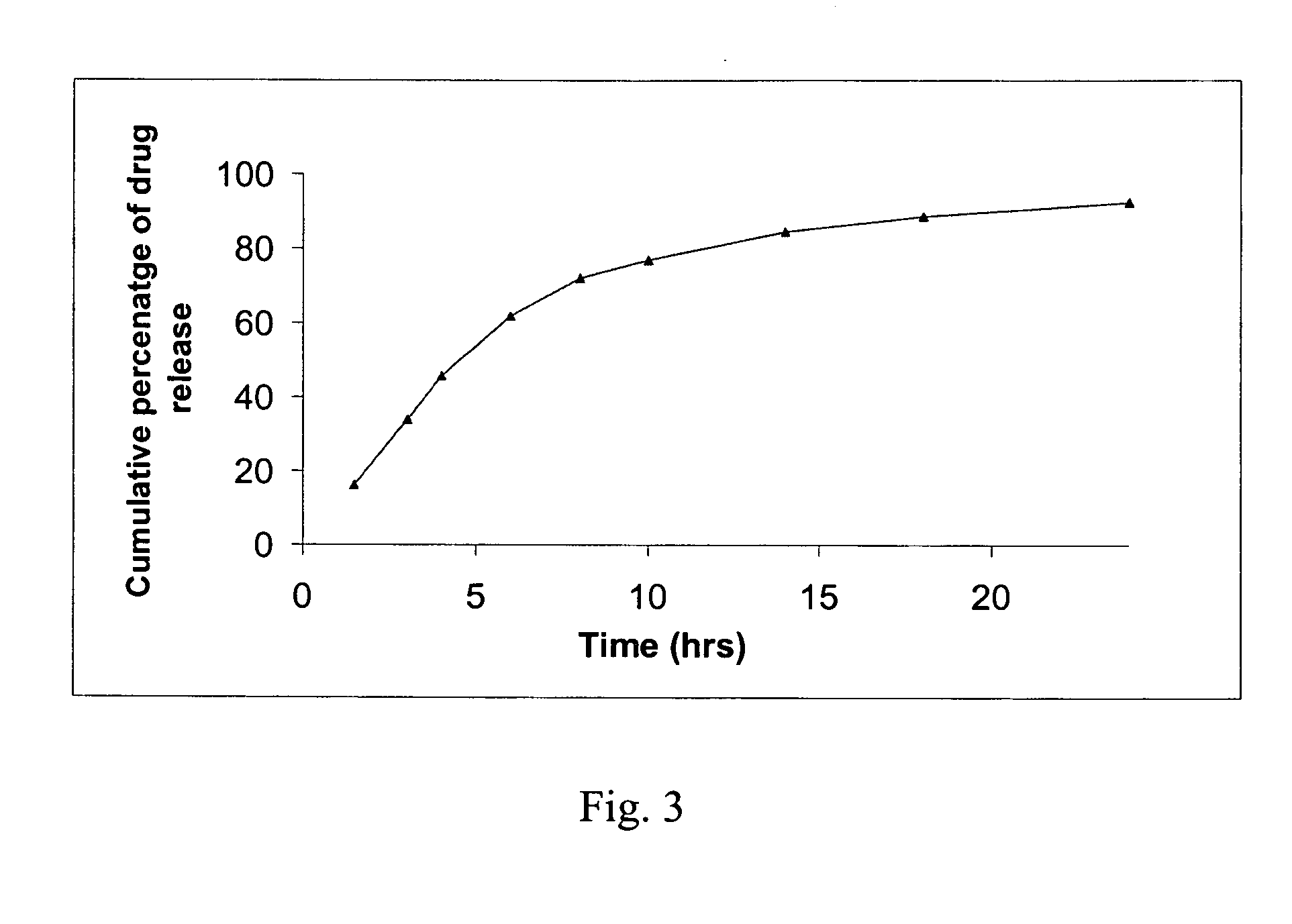
[0088] Propranolol hydrochloride 120 mg is used per dosage form which may be prepared similar to Example 1, except for the difference in dosage amount and the corresponding differences in the inactive ingredients. Alternatively, the quantity of the beads of Example 1 or 2 may be adjusted proportionately to provide 120 mg of the dose of propranolol hydrochloride.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com