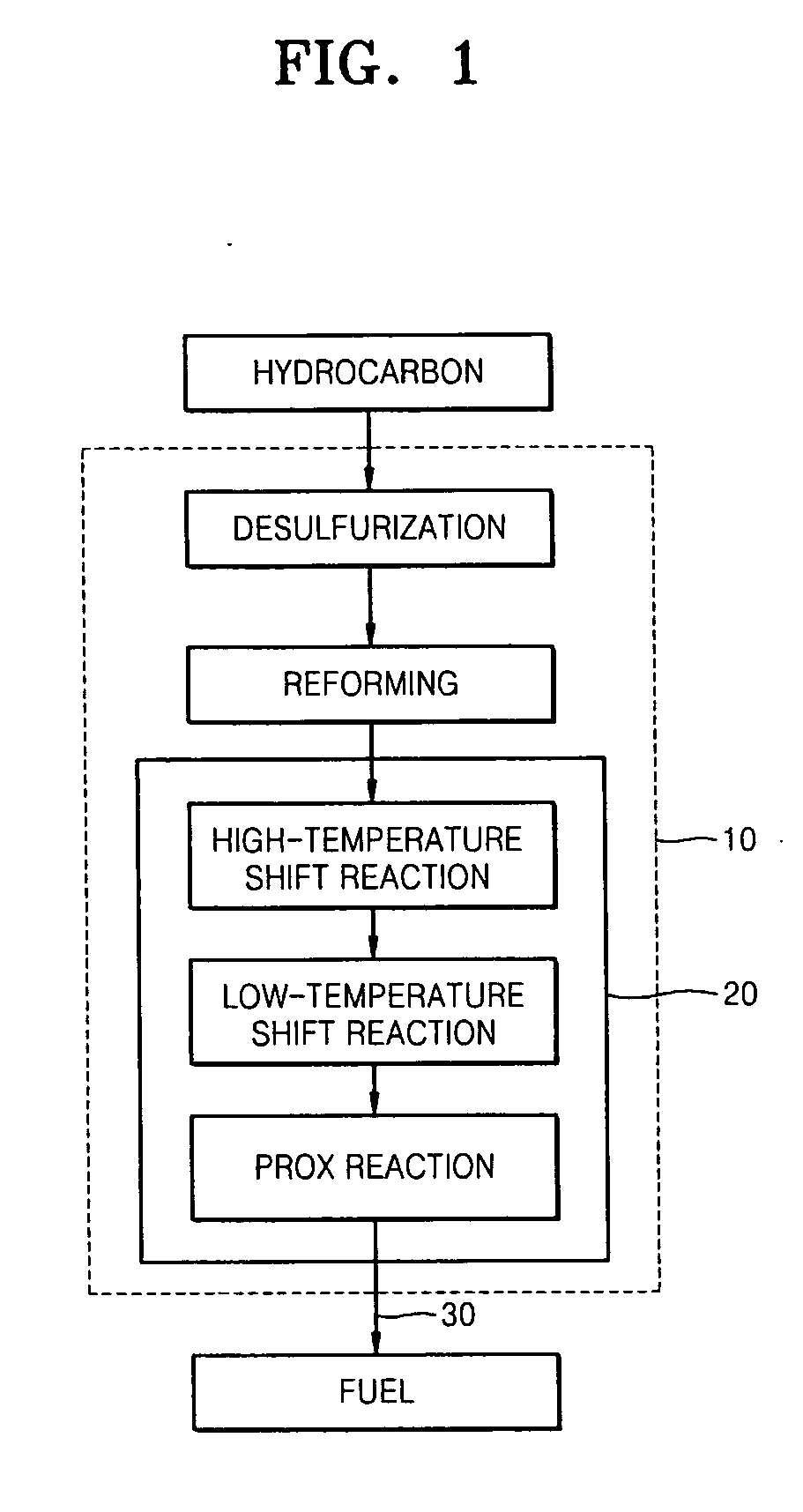
Catalyst for oxidizing carbon monoxide and method of manufacturing the same
a carbon monoxide and catalyst technology, applied in the direction of catalyst activation/preparation, metal/metal-oxide/metal-hydroxide catalysts, final product manufacturing, etc., can solve the problem of significant hydrogen concentration drop, difficulty in reducing co levels to less than 5,000 ppm, and methane reaction. , to achieve the effect of excellent reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
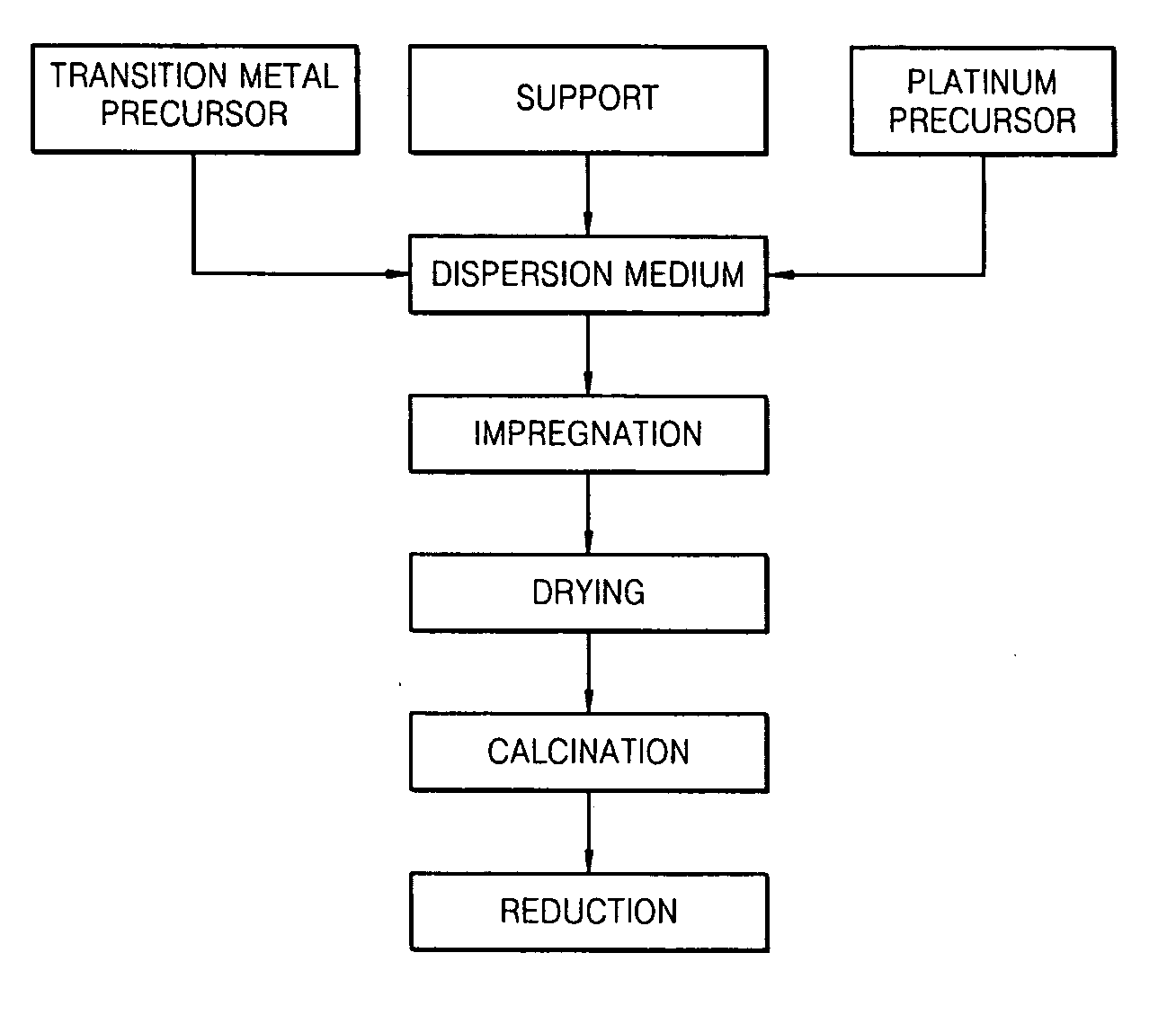
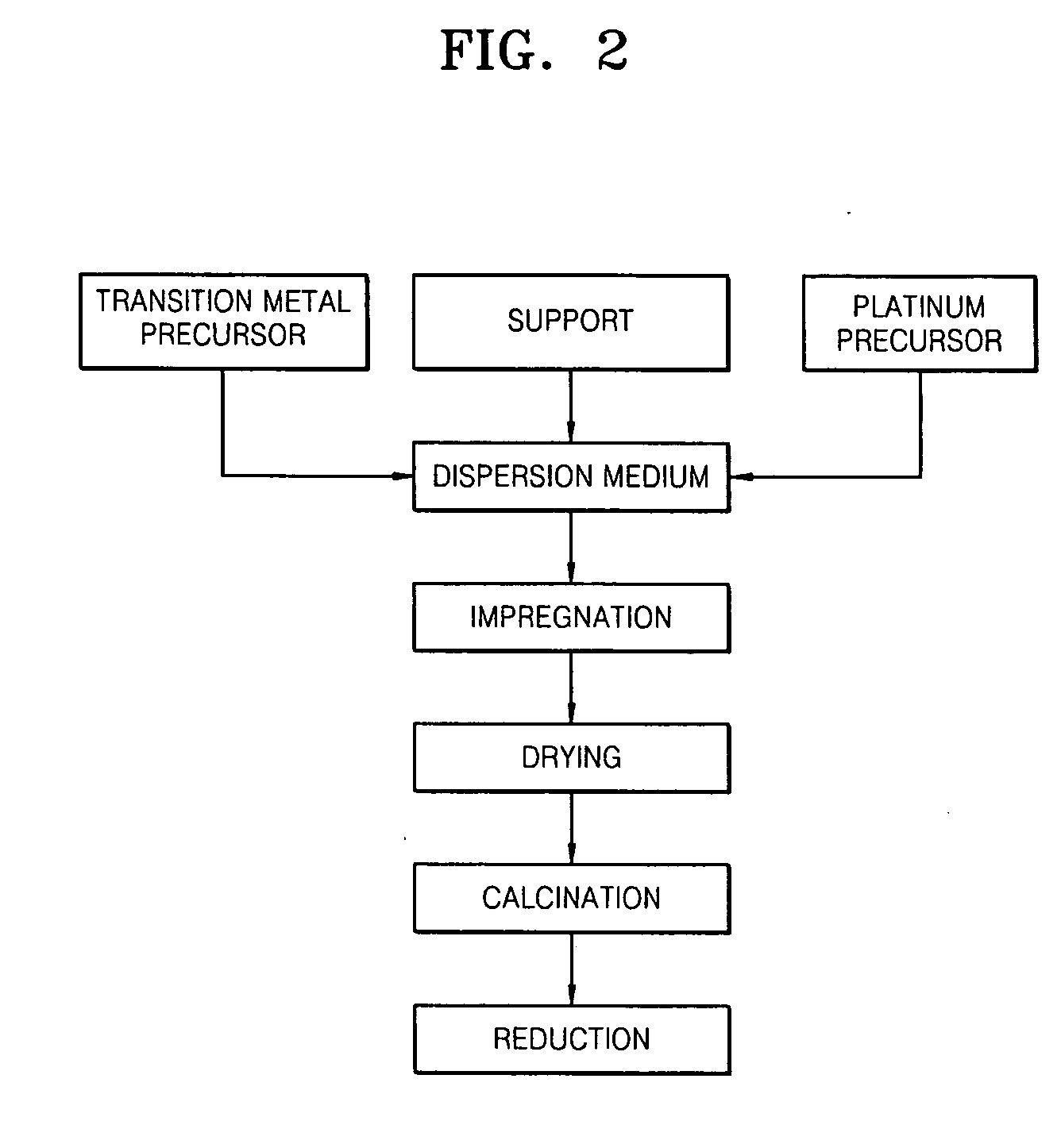
[0072] 0.207 g of Pt(NH3)4(NO3)2, 1.553 g of Ni(NO3)2.6H2O, and 10 g of γ-alumina were added to 50 ml of water and the mixture was stirred for 6 hours to prepare a uniform mixture. The mixture was dried at 60° C. in a vacuum to remove the solvent, and dried at 110° C. for 12 hours in an oven. Then, the dried resultant was calcined at 350° C. for 2 hours under an air atmosphere. The calcined resultant was reduced at 300° C. for 2 hours in an oven under a H2 atmosphere to prepare Pt—Ni / γ-Al2O3.
example 2
[0073] Pt—Co / γ-Al2O3 was prepared in the same manner as in Example 1, except that 1.554 g of Co(NO3)2.6H2O was used instead of Ni(NO3)2.6H2O.
example 3
[0074] Pt—Cu / γ-Al2O3 was prepared in the same manner as in Example 1, except that 1.004 g of Cu(NO3)2.H2O was used instead of Ni(NO3)2.6H2O.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature programmed oxidation | aaaaa | aaaaa |
| temperature programmed oxidation | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com