Therapy procedure for drug delivery for trigeminal pain
a trigeminal pain and drug delivery technology, applied in the field of pain treatment methods and compositions, can solve the problems of limiting function, reducing mobility, and ineffective treatment of pain, and achieve the effect of reducing pain and reducing pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
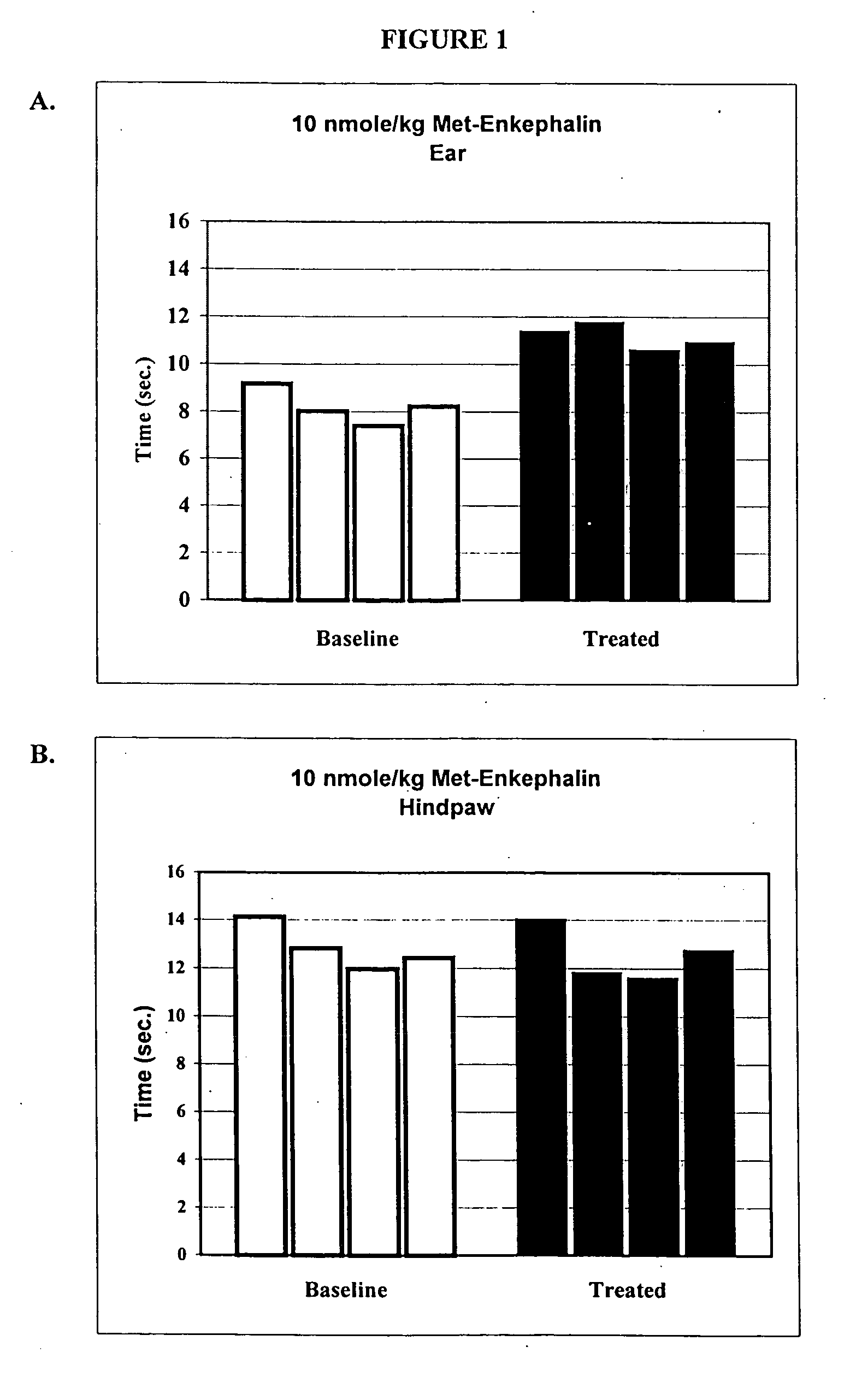
[0134] One way to test activity of an analgesic agent in a rat model is by treatment-induced changes in latencies (times) of withdrawal in response to noxious heating of the skin, typically using an ear, the face or a hindpaw. Thus, application of coherent or non-coherent (non-laser) radiant heat to the ear, the face or hindpaw will elicit rapid withdrawal movements. Latencies of withdrawal have been demonstrated to be sensitive to analgesic treatments, such that analgesics increase the latency to withdrawal. Transmucosal or transdermal administration of analgesic agents to the trigeminal nerve to reduce trigeminal nerve-associated pain can be tested for regional and / or systemic analgesia. The rostral external part of a rat's ear is innervated by a branch of the mandibular nerve, itself a branch of the trigeminal nerve, thus after treatment an increase in latency to withdrawal time would indicate regional analgesia. A change in the latency to withdrawal time of the hindpaw would ind...
example 2
[0138] Sprague-Dawley rats (Charles River Laboratories) were lightly anesthetized with urethane and placed with minimal restraint on a heating pad to maintain their body temperature at 37° C. A laser beam was directed via a fiberoptic cable to the rostral external part of both ears or to the hindpaws as described above. Baseline withdrawal latencies were measured by delivering 4 separate stimuli with a resting period of approximately 15 minutes between each stimulus. 50 μl of met-enkephalin in phosphate-buffered saline was intranasally administered in 5 equal 10 μl applications at a dosage of 10 nmoles / kg of body weight. Withdrawal latencies for both ears and hindpaws were tested five minutes after the final application of met-enkephalin. As described above, testing sessions were videotaped and analyzed. Results demonstrated that intranasal administration of met-enkephalin at this dosage achieved a regional analgesic effect in the head region (FIG. 1A) without a systemic analgesic e...
example 3
Normal Human Volunteers.
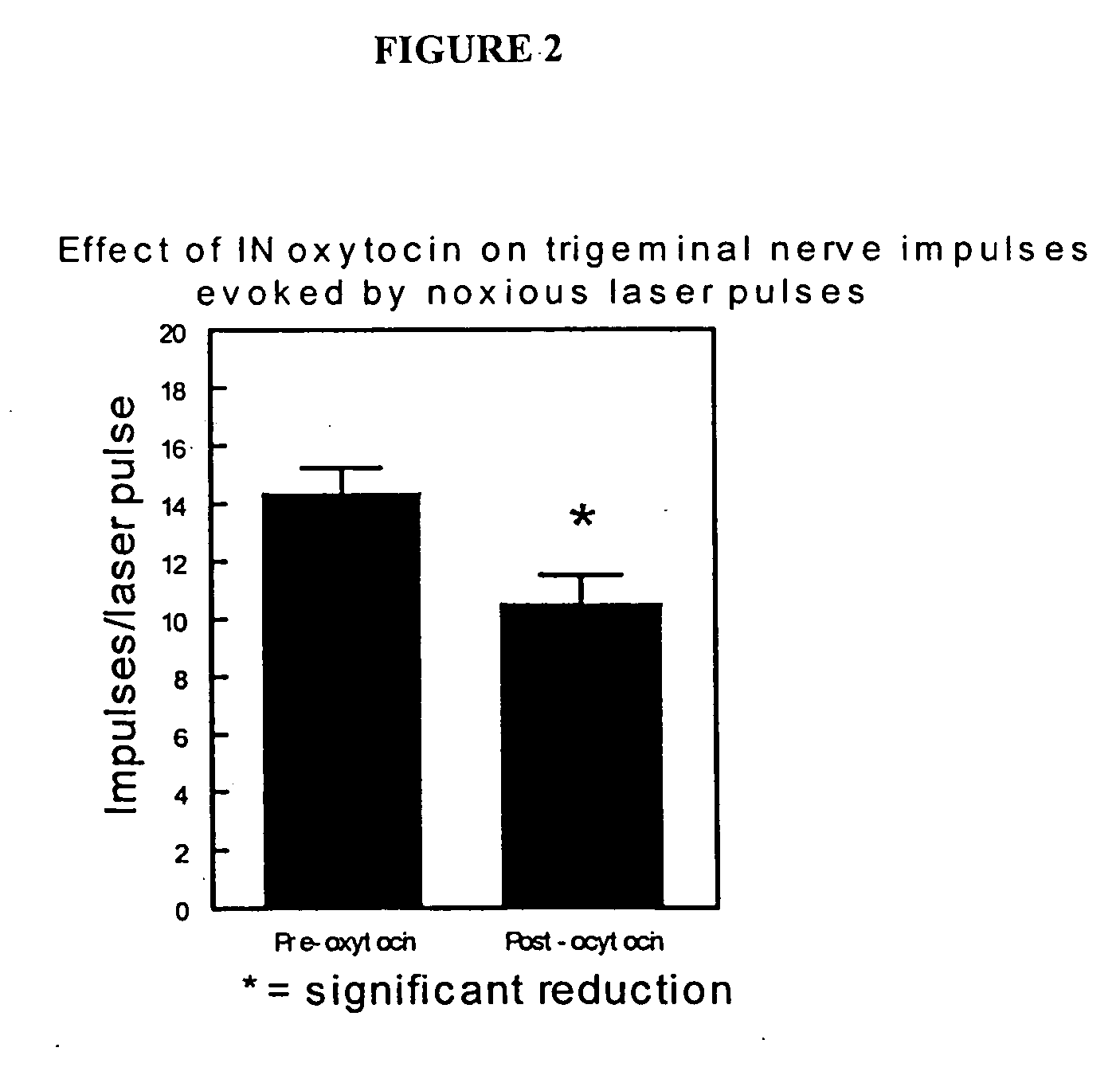
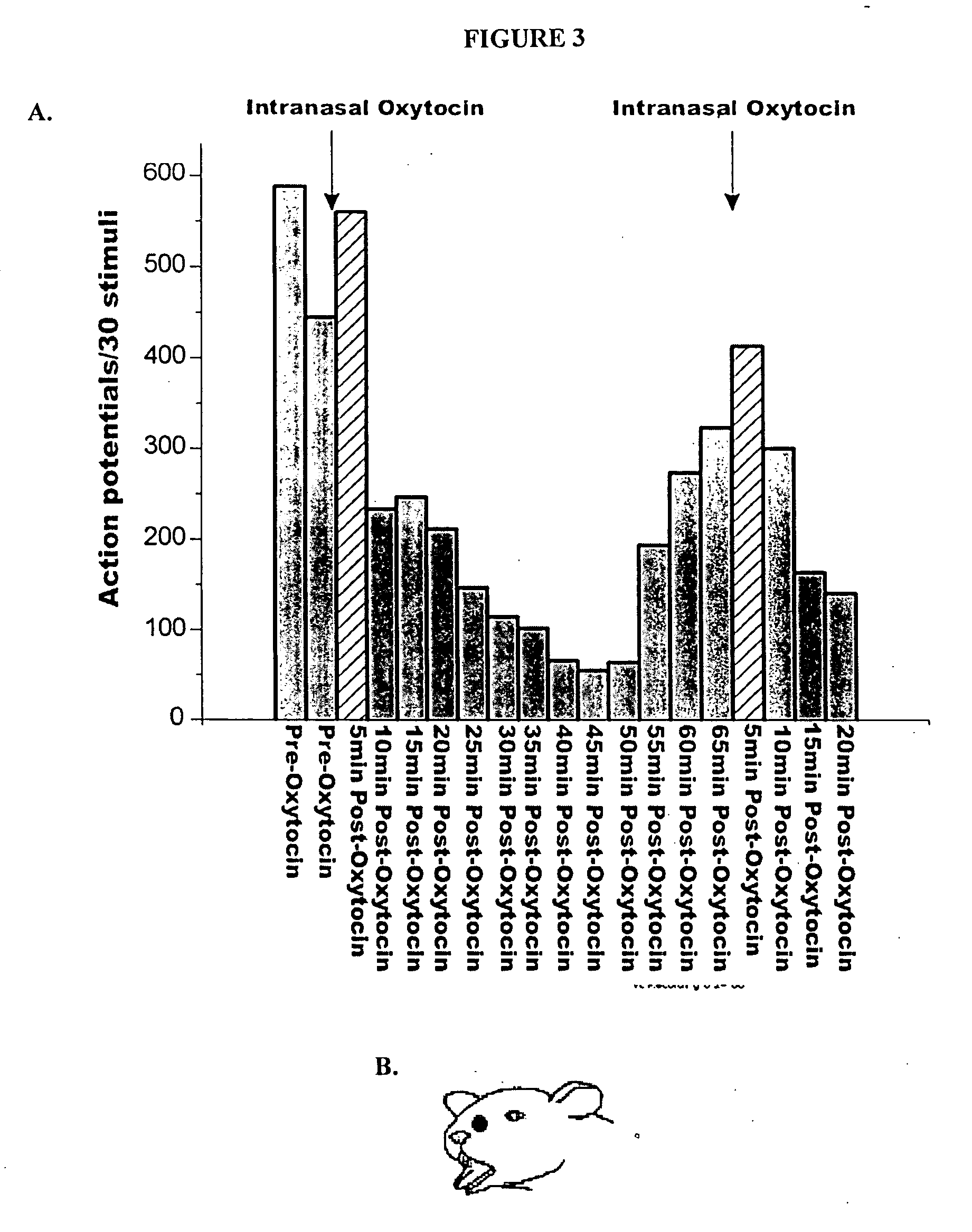
[0139] Regional analgesia in the face region after administration of analgesic agents by intranasal delivery can be tested in normal subjects. Study participants are selected based on inclusiori / exclusion criteria, history and physical exam, laboratory tests, and other customary procedures. Thermal pain responses are elicited on the face, in particular the cheek, and on the hand of healthy normal volunteers, such that temperature thresholds for evoking pain and / or the temperature of maximal pain tolerance can be assessed and baselines established. Increasing doses of an analgesic agent are administered to the subjects and a dose-response curve is calculated for each stimulation site. Changes in thermal pain threshold and tolerance at the two sites can be compared so that the efficacy of an analgesic agent at a given dose in affecting facial and whole-body pain can be determined.
[0140] The analgesic agent is delivered intranasally to the subjects by a meter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| distance | aaaaa | aaaaa |
| distance | aaaaa | aaaaa |
| body temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com