Methods and devices for improving cardiac function in hearts
a heart and cardiac function technology, applied in the field of apparatus for treating a failing heart, can solve the problems of significant increase in wall tension and/or stress, dilatation of the left ventricular chamber, and inability to achieve the effect of reducing the radius of curvature of the heart wall
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 116
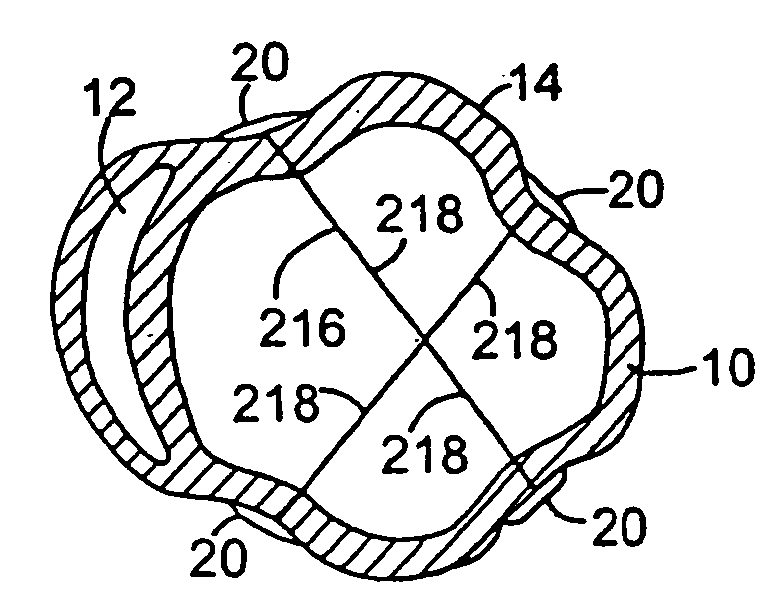
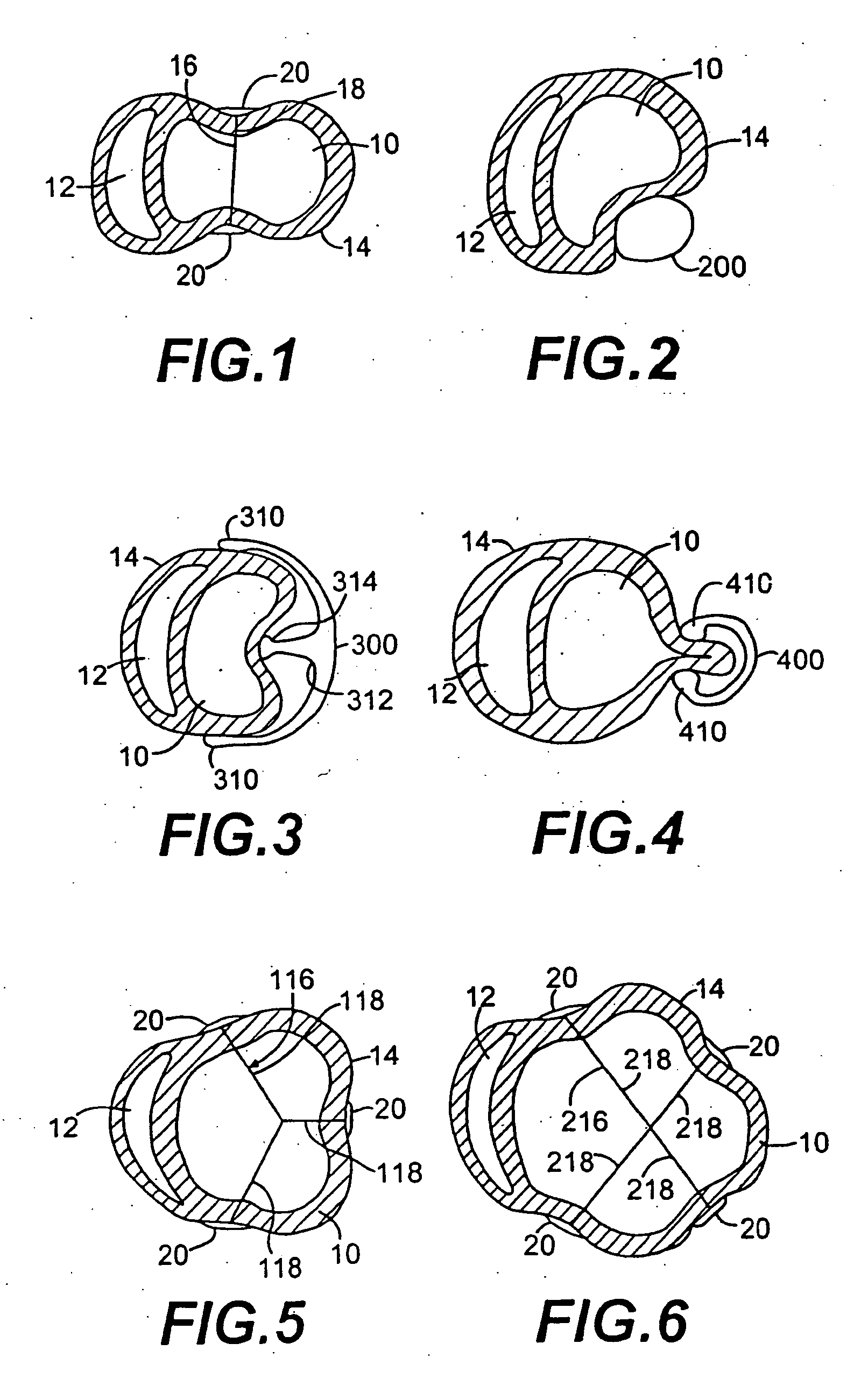
[0125]FIG. 5 shows an alternate embodiment of the splint of FIG. 1 referred to in FIG. 5 by the numeral 116. The embodiment 116 shown in FIG. 5 includes three tension members 118 as opposed to a single tension member 18 as shown in FIG. 1. FIG. 6 shows-yet another embodiment of the splint 216 having four tension members 218. It is anticipated that in some patients, the disease process of the failing heart may be so advanced that three, four or more tension members may be desirable to reduce the heart wall stresses more substantially than possible with a single tension member as shown in FIG. 1.
embodiment 316
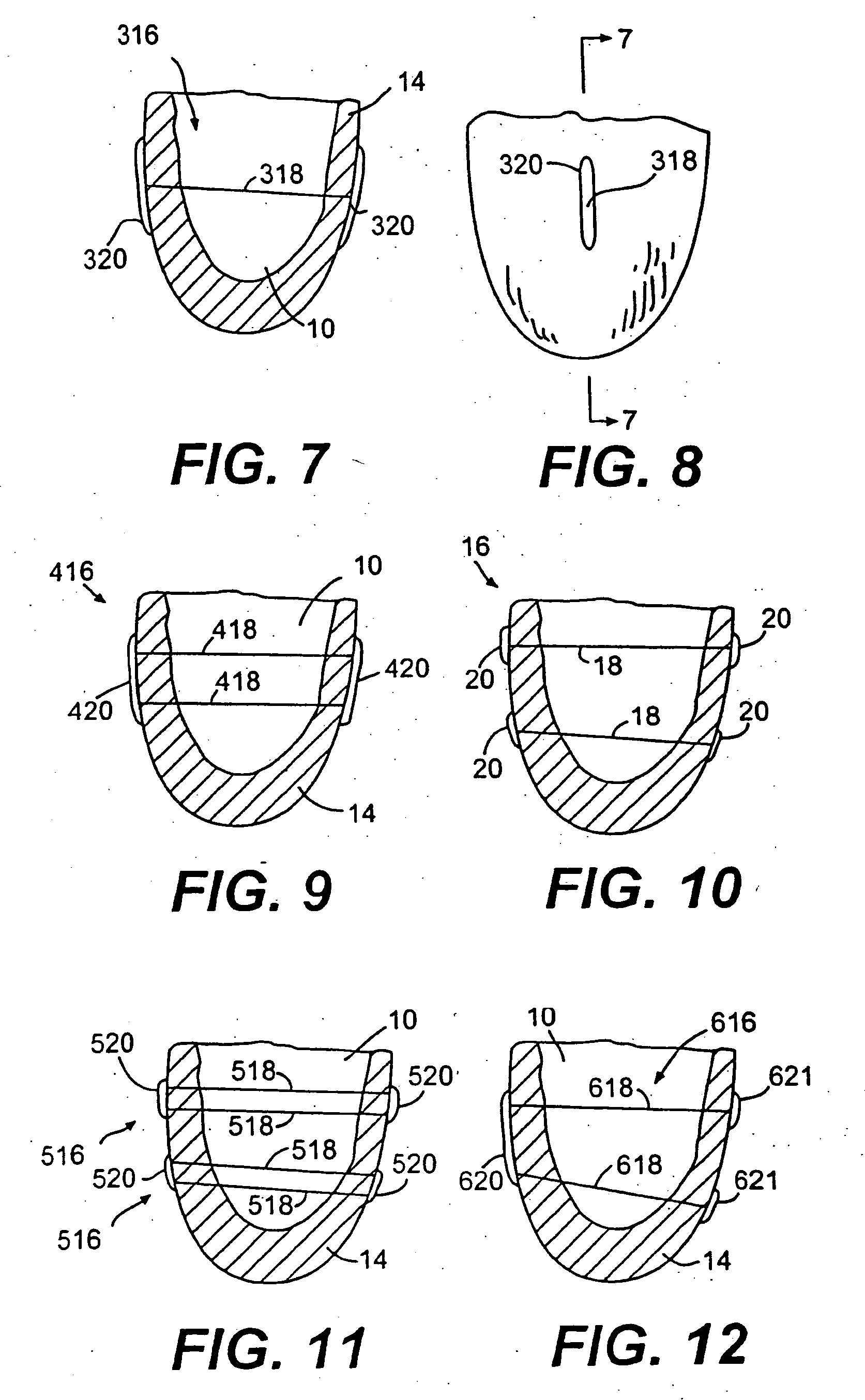
[0126]FIG. 7 is a partial vertical cross-section of human heart 14 showing left ventricle 10. In FIG. 7, another splint embodiment 316 is shown having a tension member 318 extending through left ventricle 10. On opposite ends of tension member 318 are disposed elongate anchors or pads 320. FIG. 8 is an end view of tension member 318 showing elongate anchor 320.
[0127]FIG. 9 shows another embodiment of a splint 416 disposed in a partial vertical cross-section of human heart 14. Splint 416 includes two elongate anchors or pads 420 similar to those shown in FIGS. 7 and 8. In FIG. 9, however, two tension members 418 extend through left ventricle 10 to interconnect anchors 420 on opposite sides of heart 14.
[0128]FIG. 10 is a vertical cross section of heart 14 showing left ventricle 10. In this case, two splints 16 are disposed through left ventricle 10 and vertically spaced from each other to resemble the configuration of FIG. 9.
[0129]FIG. 11 is a vertical cross-sectional view of the le...
embodiment 156
[0132]FIG. 14 shows an alternate embodiment 156 of the splint shown in FIG. 13. In this case lever members 154 are longer than members 54 as compression member 152 of splint 150 has been disposed to the exterior of left ventricle 10.
[0133]FIG. 15 is a vertical cross sectional view of left ventricle 10 of heart 14. An alternate embodiment 250 of the splint is shown on heart 14. A preferably relatively rigid frame member 256 extends through ventricle 10. Disposed on opposite ends of frame 256 are cantilever member 254. Disposed on cantilever members 254 are atraumatic pads 258. Cantilever members 254 can be positioned along frame member 256 such that atraumatic pads 258 press against heart 14 to compress chamber 10. FIG. 16 is an end view of frame member 256 showing cantilever members 254 and pads 258.
[0134] It should be understood that each of the embodiments described above should be formed from suitable biocompatible materials known to those skilled in the art. The tension members...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com