Endoluminal implant with therapeutic and diagnostic capability
a technology of endoluminal implants and diagnostic capabilities, which is applied in the field of endoluminal implants with, implantable medical devices, can solve the problems of inability to supply power to such sensors, use of conventional sensors, and inability to solve problems such as practical solutions, so as to facilitate localized drug delivery, prevent further tissue growth, and improve the effect of medical treatment of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
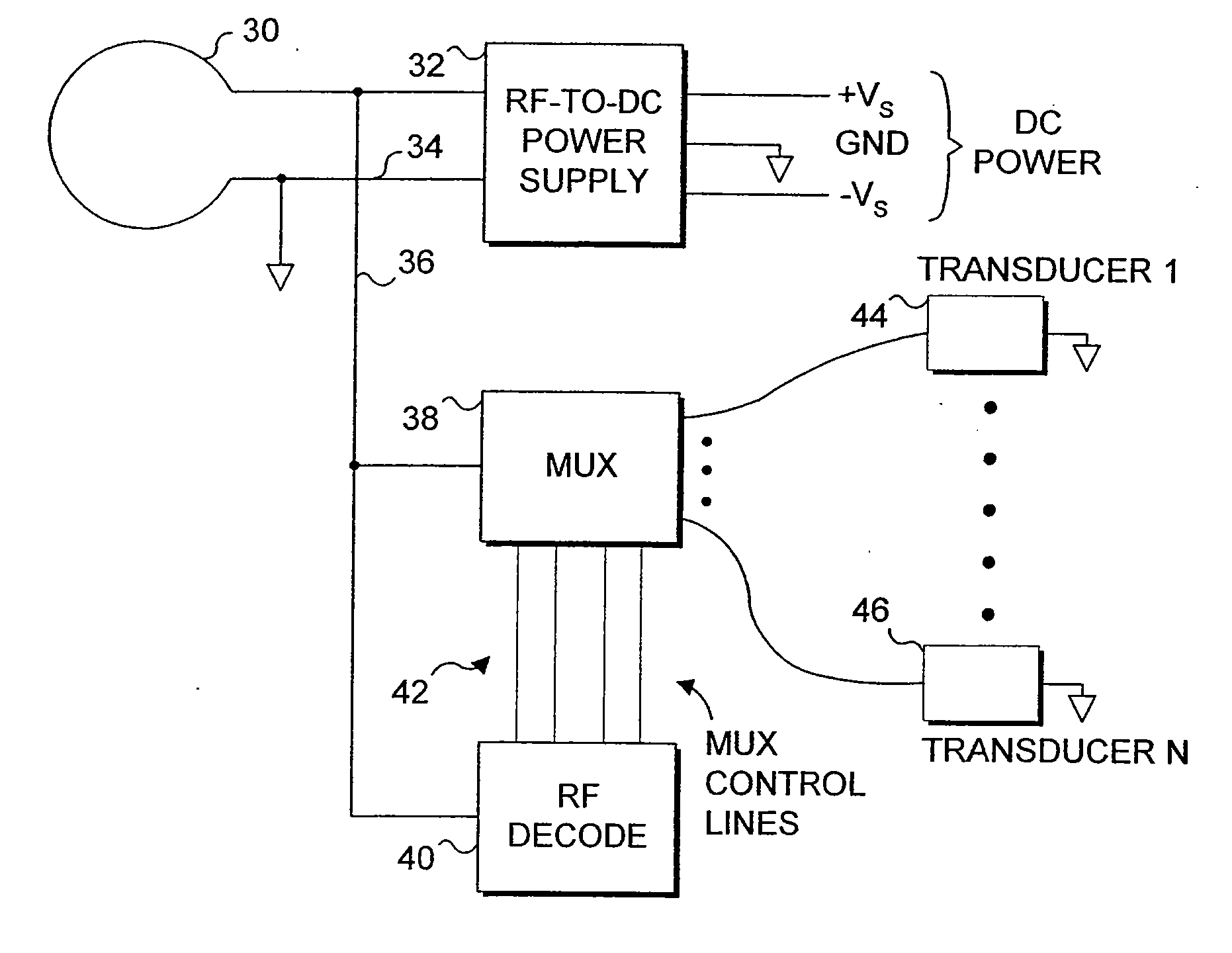
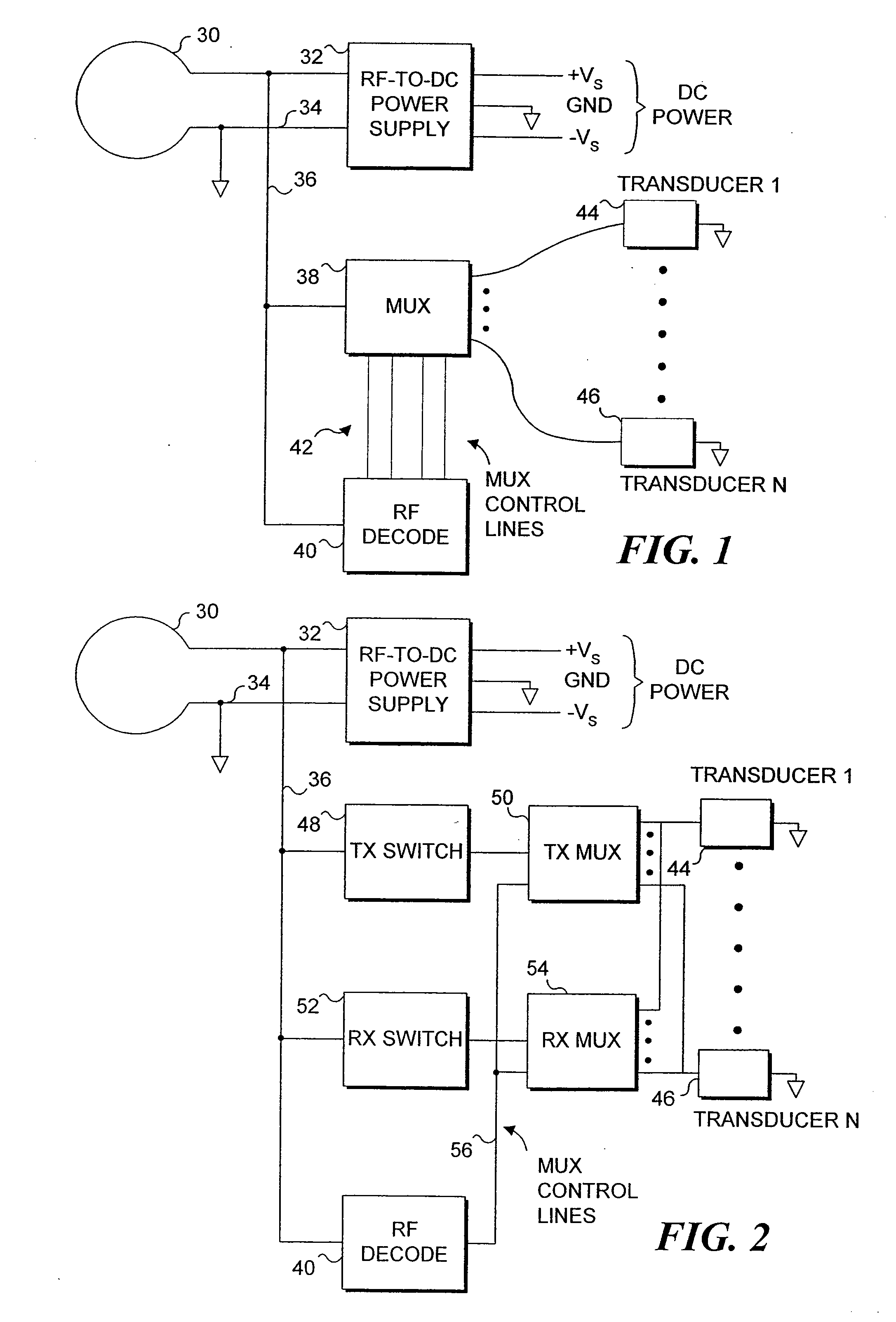
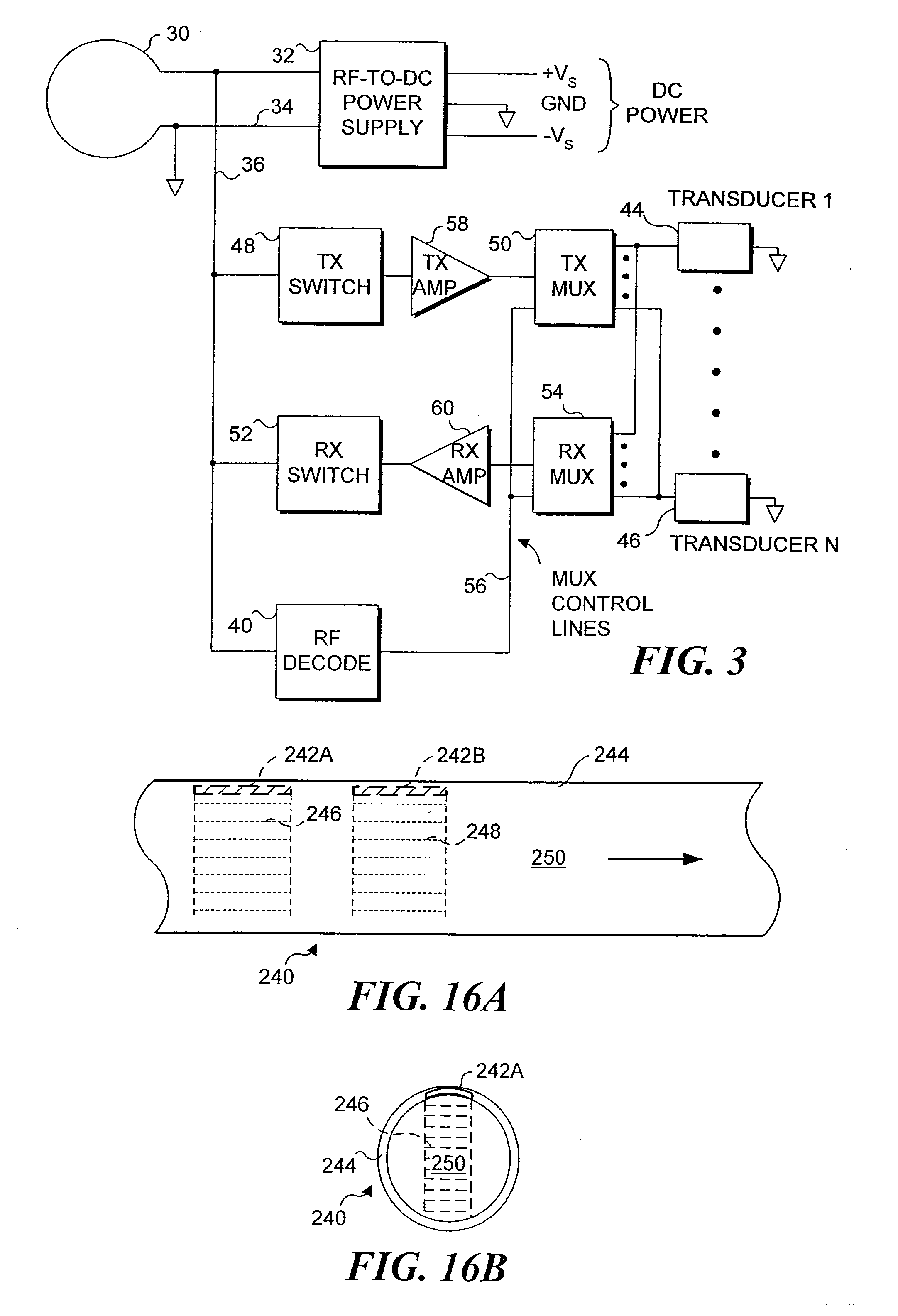
[0050] The present invention is employed for providing therapeutic functions proximate to an endoluminal implant. As used herein and in the claims that follow, the term endoluminal implant broadly encompasses stents, stent grafts (sometimes referred to as “spring grafts”) and other types of devices that are inserted into a lumen or body passage and moved to a desired site to provide a structural benefit to the lumen. To simplify the disclosure of the present invention, most of the following discussion is directed to embodiments comprising a stent.
[0051] In one embodiment, parameters are monitored via implanted diagnostic transducers, where the monitored parameters are directed to determining the status of the fluid flow through the endoluminal implant, and therapeutic transducers may be activated in response to the data collected from the implanted diagnostic transducers. For example, the rate or velocity of fluid flow through a body passage in which the stent has been positioned c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com