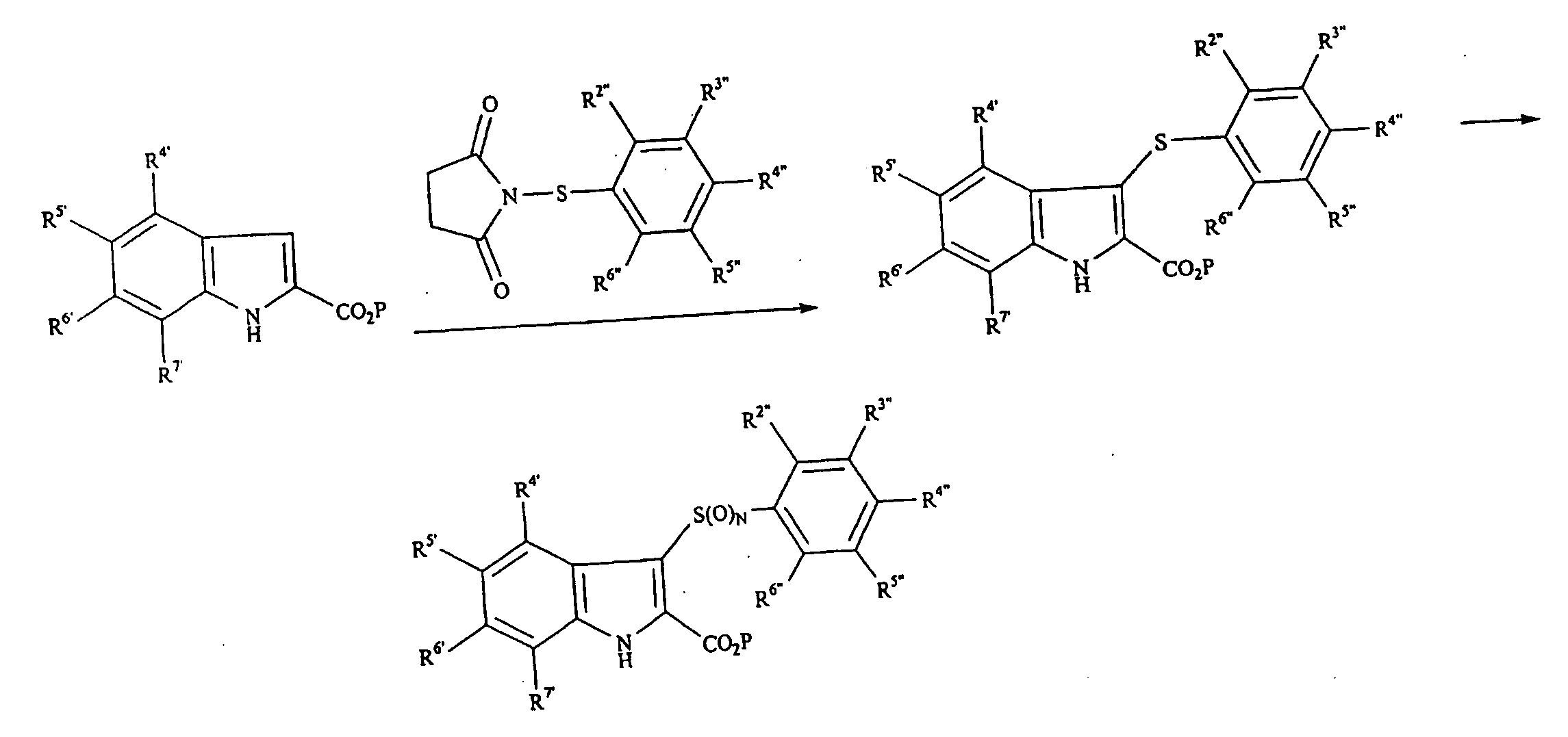
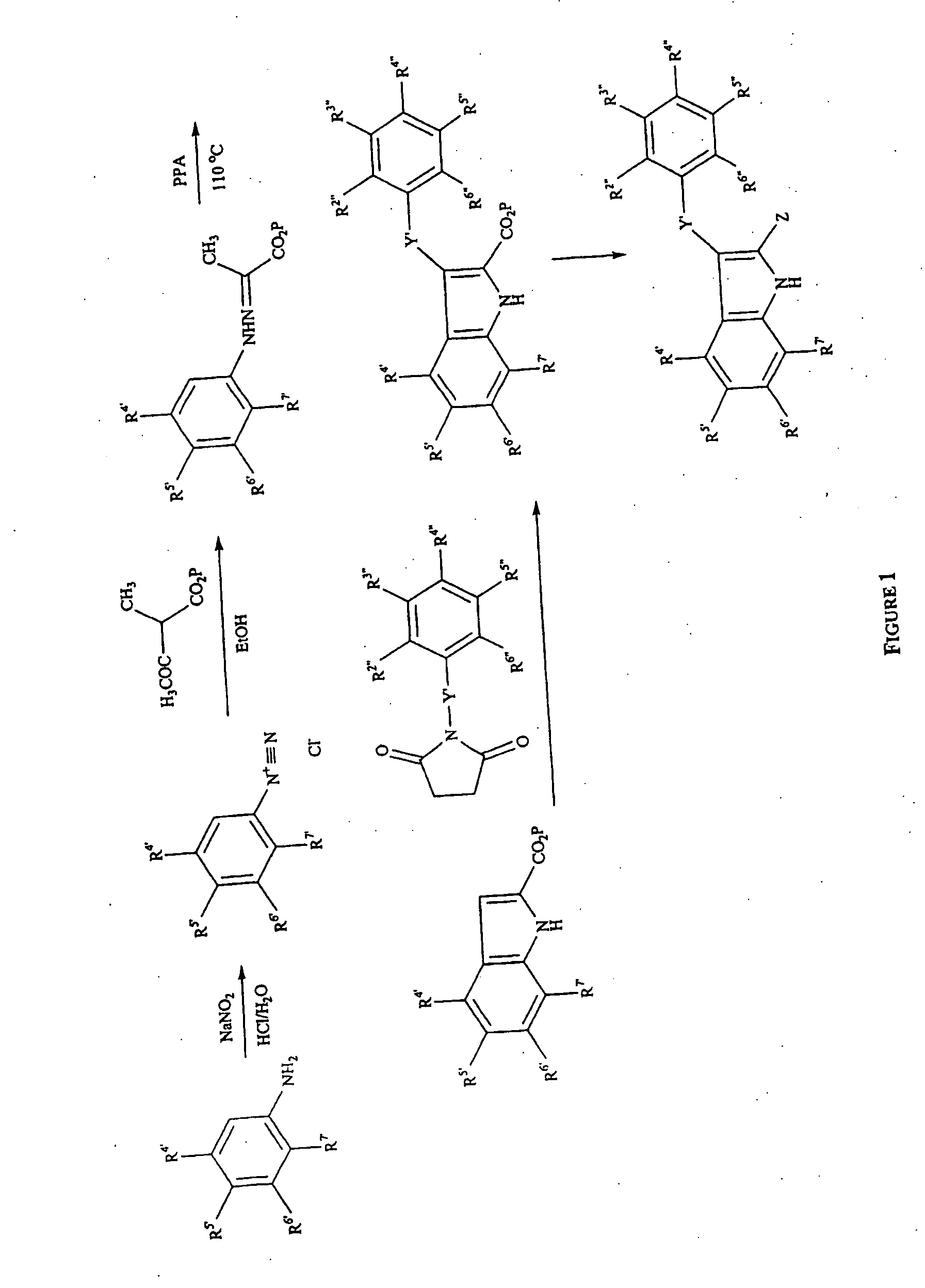
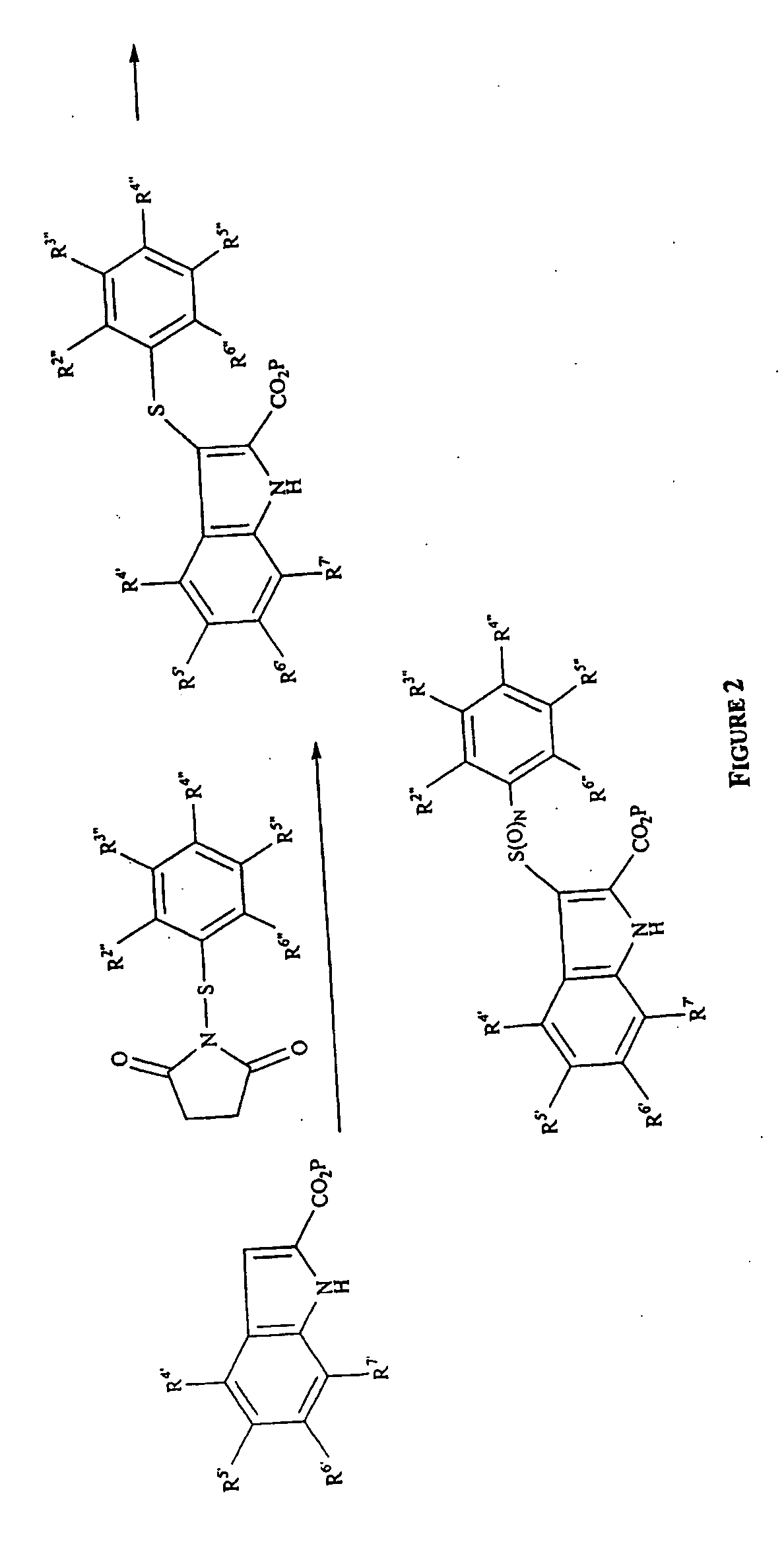
Phenylindoles for the treatment of HIV
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Ethyl Pyruvate 4-Chloro-3-Fluorophenylhydrazone
[0449] A solution of sodium nitrite (4.76 g, 0.069 mol) in water (6.3 mL) was added dropwise to an ice cooled mixture of 4-chloro-3-fluoroaniline (J. Am. Chem. Soc., 1996, 61, 5130-5133) (10.00 g, 0.069 mol), water (167 mL) and 37% hydrochloric acid (167 mL). After 20 minutes potassium acetate (9.81 g, 0.10 mol) was added, and then a solution of ethyl 2-methylacetoacetate (9.95 g, 0.069 mol), potassium acetate (9.81 g, 0.10 mol) in methanol (67 mL) was dropped while cooling on the ice bad. Reaction was stirred at 0° C. for 3 hours, then extracted with diethyl ether. Organic layer was washed with brine and dried. Removal of the solvent furnished a red oily residue that was treated with ethanol (100 mL) and stirred at room temperature overnight. The solid which formed was filtered a recrystallized from ethanol to give 5.4 g (30%) of title compound, mp 161-163° C. (from ethanol). [0450] Ethyl pyruvate 2,4-difluorophenylhydraz...
example 2
Synthesis of Ethyl pyruvate 2,4-dichlorophenylhydrazone
[0453] A mixture of 2,4-dichlorophenylhydrazine (16.00 g, 0.075 mol), ethyl pyruvate (14.47 g, 10.3 mL, 0.12 mol), glacial acetic acid (0.9 mL), absolute ethanol (105 mL) was refluxed for 2 hours. After cooling at room temperature, the solid which formed was filtered and recrystallized from ethanol to give 17.0 g (83%) of the title compound, mp 118-120° C. (from ethanol).
example 3
Synthesis of Ethyl 5-chloro-6-fluoroindole-2-carboxylate (2j) and ethyl 5-chloro-4-fluoroindole-2-carboxylate (2 g)
[0454] Ethyl pyruvate 4-chloro-3-fluorophenylhydrazone (5.00 g, 0.0193 mol) was added by portions to PPA (50 g) pre-heated at 110° C., then reaction was stirred for 30 minutes. After cooling at room temperature, ice water was added while stirring. The solid which formed was filtered, washed with water, dried and passed by a silica gel column chromatography (n-hexane:ethyl acetate 1:2 as eluent). First fractions furnished ethyl 5-chloro-6-fluoroindole-2-carboxylate (2f), (1.85 g, 40%), mp 160-164° C.° (ethanol). Further elution with the same eluent gave ethyl 5-chloro-4-fluoroindole-2-carboxylate (2 g) (0.9 g, 19%), mp 186-190° C. (ethanol). [0455] Ethyl 5,7-dichloroindole-2-carboxylate (2b), yield 37%, mp 143-145° C. (from ethanol). [0456] Ethyl 4,5-difluoroindole-2-carboxylate (2c), yield 15%, mp 166-168° C. (from ethanol). [0457] Ethyl 4,5-difluoroindole-2-carboxylat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com