Ozonolysis of carbon nanotubes
a carbon nanotube and ozonolysis technology, applied in the field of ozonolysis of carbon nanotubes, can solve the problems of not being able to economically scale the previously discussed electric arc discharge and laser deposition methods cannot be economically scaled up for such commercial or industrial production, and the simultaneous destruction of carbon nanotubes or carbon nanotube structures themselves. , the effect of enhancing electrochemical characteristics and reducing the number of steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0150]Ozone was generated via an air purifier made by Del Industry, San Luis Obispo, Calif., which can generate ozone at a rate of 250 mg / hr. A mixture of ozone and air (0.29% ozone) at a flow rate of 1200 mL / min was then passed though a 1-inch (OD) reactor tube packed with dry as-made fibrils. The weight of fibrils before and after ozone treatment were recorded. The reaction was allowed to proceed for a period of 3 to 45 hours at room temperature.
[0151]In a separate experiment, 20 grams of as-made fibrils were placed in a flask containing 500 mL 30% or 60% nitric acid. The reaction flask was then heated to reflux temperature of 95-120° C. for 4-6 hours. After the reaction was stopped, the fibrils were cooled to room temperature, filtered, and washed with water until neutral. In another separate experiment, 20 grams of as-made fibrils were placed in a flask containing 376.2 grams of 30% H2O2 in molar ratio of 1:2. The temperature was set to be in the vicinity of 35° C., but it rose ...
example 2
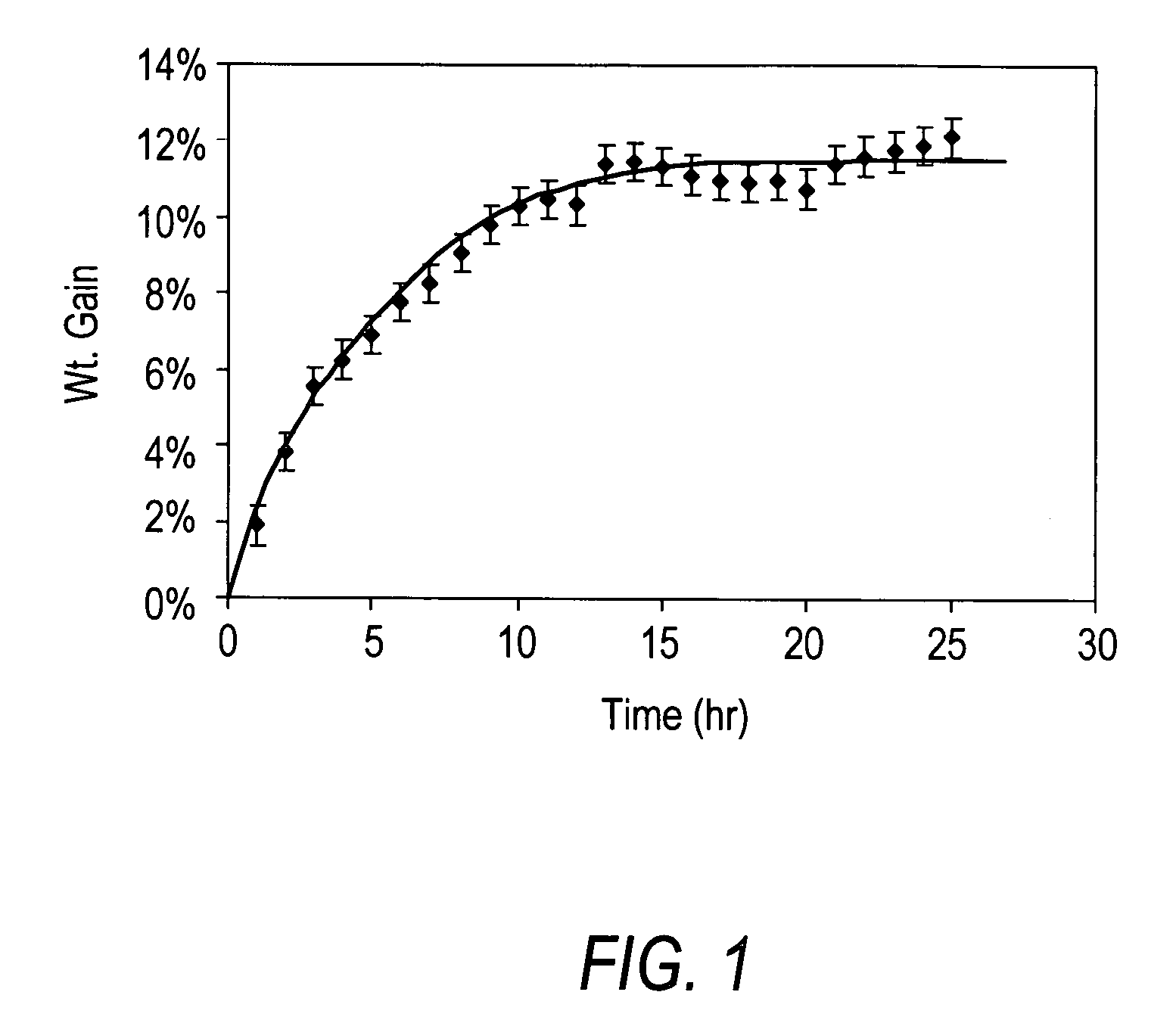
[0155]3 grams of dry fibrils was placed in a vertical reactor and ozone-containing air was passed through them at room temperature. The reactor was shut off periodically at every hour and the total weight of fibrils plus the reactor tube were measured on an electric balance. The weight gain of fibrils against reaction time was then obtained after tarring off the reactor weight.
[0156]The results of this measurement are displayed in FIG. 7, which shows that the sample's weight increased over the course of reaction and leveled off after approximately 15 hours of reaction lime.
example 3
[0157]Various oxidized fibrils prepared according to the methods in Example 1 such as Samples 1-3 and 5-7 were measured to determine their relative amount of acidic groups through titration. 0.25 gram of each sample was placed into a flask containing 300 mL D.I. water and the slurry was titrated with 0.1N NaOH. The consumption of NaOH was translated into the quantity of total surface acidic groups as meq / g.
TABLE 3Measurement of surface acidic groups through titration.Sample weightWeight changeTiterSample(g)Oxidant(%)(meq / g)13O3 / air13.951.4523O3 / air8.01.07310O3 / air10.401.5753O3 / air12.121.1682030% HNO3−15.28 1.2062060% HNO3−6.350.5872030% H2O2−4.0 0.128
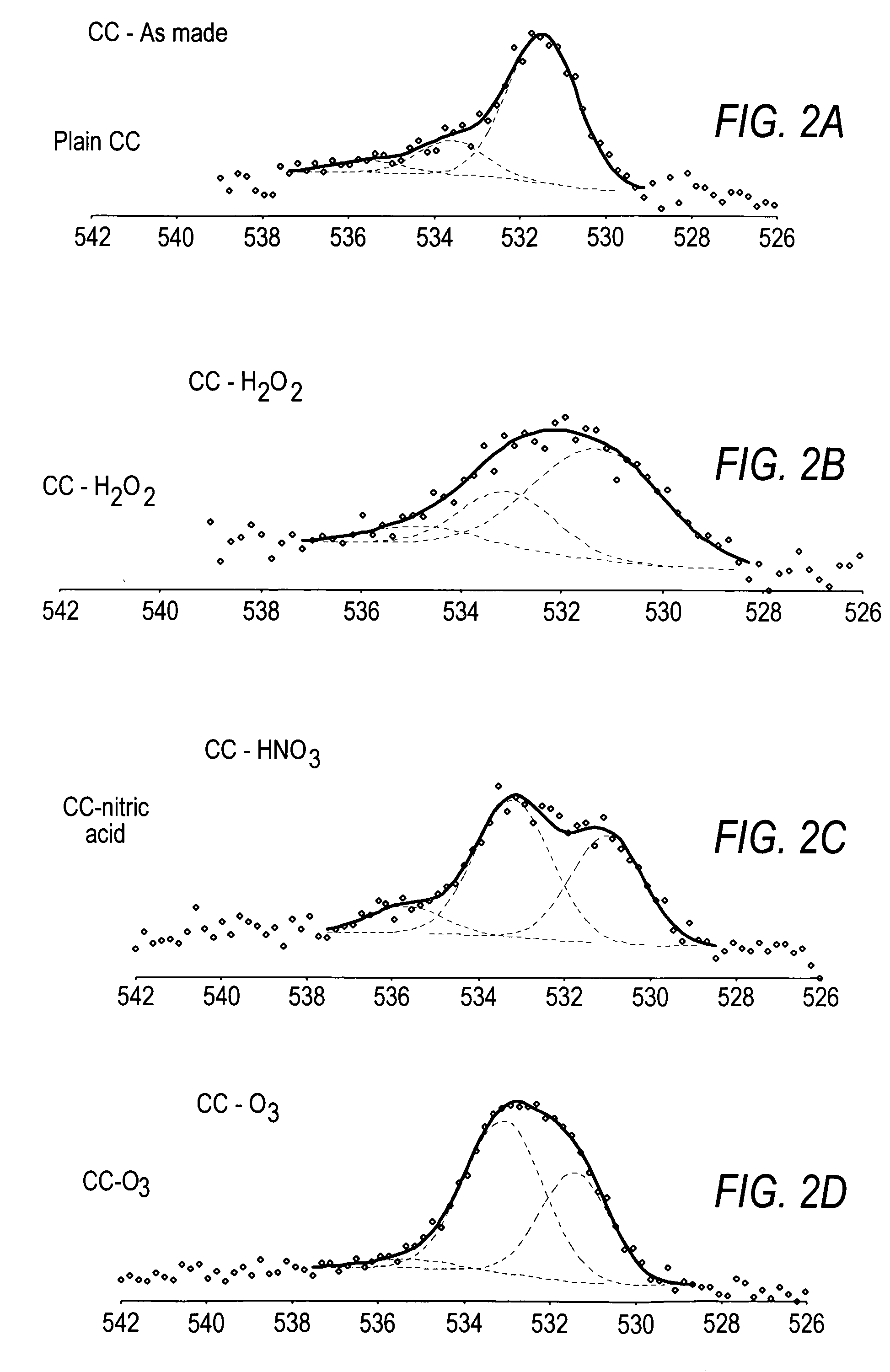
[0158]As shown in Table 3, more acidic groups can be deposited onto the surface of carbon nanotubes when oxidized with ozone at room temperature than with other oxidizing agents such as nitric acid or hydrogen peroxide. In other words, treatment of carbon nanotubes with ozone at room temperature will yield nanotubes with a higher titer ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com