Method of formation of shape-retentive aggregates of gel particles and their uses
a gel particle and aggregate technology, applied in the field of shape-retentive aggregate formation of gel particles, can solve the problems of difficult control of particle dissemination to, inability to localize at, and inability to control the diffusion of particles to, currently available particulate gels, and achieve the effects of reducing the risk of fracture, and improving the stability of the gel particl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Hydrogel Particle Preparation using Tween 80 Surfactant
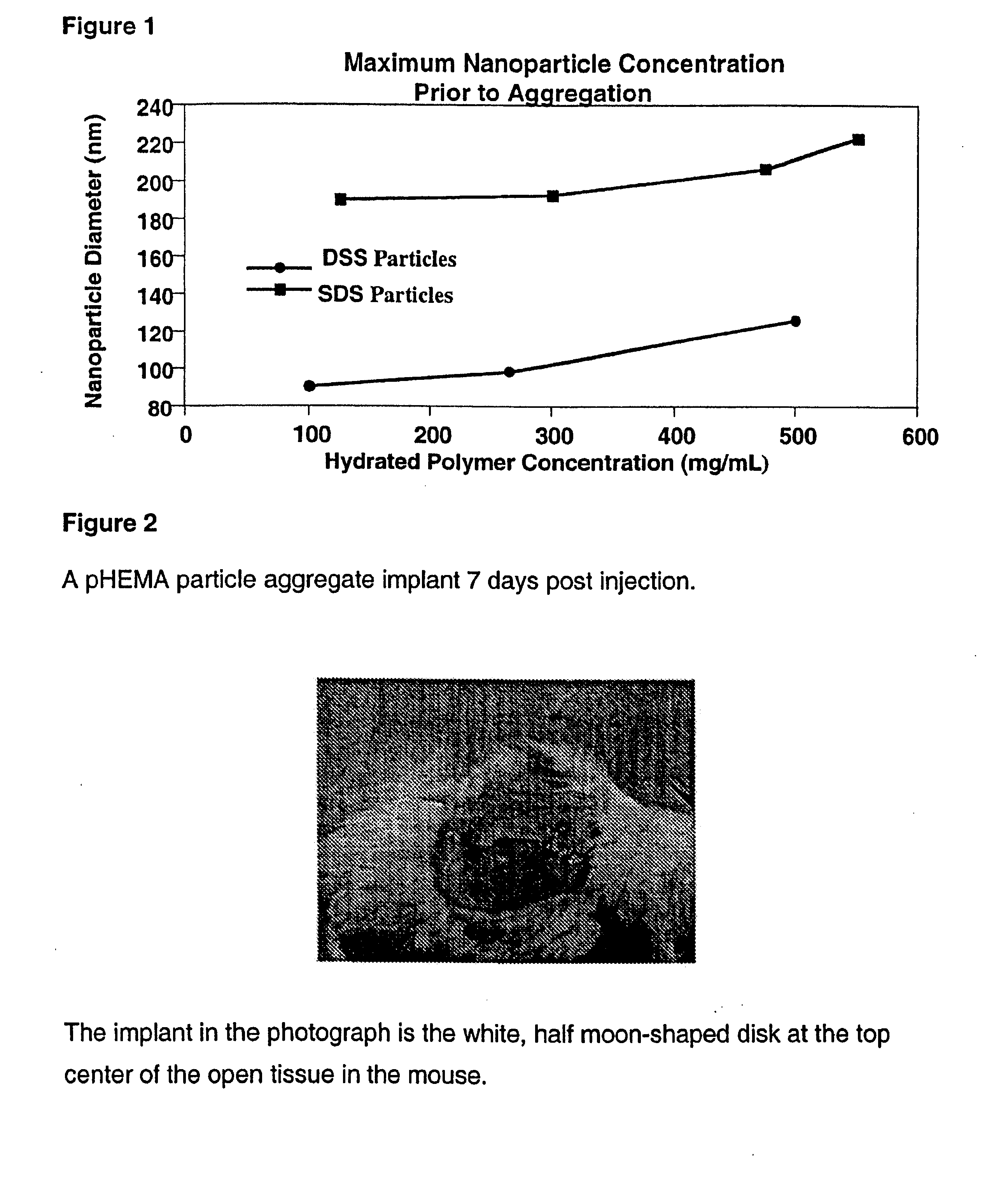
[0215] A stock solution of Tween 80 in Milli-Q H2O was prepared by dissolving 27 g of Tween (80) with 100 g of Milli-Q H2O. A stock solution of potassium persulfate was prepared by dissolving 2 g in 30 mL of Milli-Q H2O. A 1 L media bottle was equipped with a stir bar and charged with 1.74 g HEMA containing 3.6 mg ethylene glycol dimethacrylate, 1.07 g of the Tween 80 stock solution, 571 mL of N2 purged Milli-Q H2O, and 0.952 mL of the potassium persulfate stock solution. The solution was stirred until all of the solids dissolved. The bottle was covered with foil and immersed in a 65° C. bath for 16 hours. The resulting suspension of hydrogel particles had a milky-white to opaque blue opalescence and the particles were found to have an average diameter of 466 nm by dynamic light scattering. The suspension was concentrated and purified by tangential flow filtration and was found to be stable against flocculation at a wet weight ...
example 2
Hydrogel Particle Preparation Using Dioctyl Sodium Succinate Surfactant
[0216] A 500 mL media bottle equipped with a stir bar was charged with 4.25 g HEMA monomer containing 8.8 mg ethylene glycol dimethacrylate, 290.18 mg dioctyl sodium succinate (DSS), 135 mg potassium persulfate, and 500 mL of N2-purged Milli-Q H2O. The bottle was capped and the solution was stirred for 3 hrs at room temperature. The bottle was transferred to a 50° C. water bath and incubated for 12 hrs. The resulting suspension of hydrogel particles had an opalescent blue color. The particles were analyzed by dynamic light scattering and found to have an average diameter of 241 nm. The suspension of particles contained 25 mg of hydrated polymer per mL of solution. The suspension was concentrated and purified using tangential flow filtration and was found to be stable against flocculation at a wet weight concentration approaching 150 mg / mL.
example 3
Hydrogel Particle Preparation Using Sodium Dodecyl Sulfate Surfactant
[0217] A 100 mL media bottle equipped with a stir bar was charged with 4.25 g HEMA monomer containing 8.8 mg ethylene glycol dimethacrylate, 267.91 mg sodium dodecyl sulfate (SDS), 135 mg potassium persulfate, and 500 mL of N2-purged Milli-Q H2O. The bottle was capped and the solution was stirred for 3 hrs at room temperature. The bottle was transferred to a 50° C. water bath and incubated for 12 hrs. The resulting suspension of hydrogel particles had an opalescent blue color. The particles were analyzed by dynamic light scattering and found to have an average diameter of 110 nm. The suspension of particles contained 78 mg of hydrated polymer per mL of solution. The suspension was concentrated and purified using tangential flow filtration and was found to be stable against flocculation at a wet weight concentration approaching 200 mg / mL.
PUM
| Property | Measurement | Unit |
|---|---|---|
| mole percent | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com