Composition and method for treating cancer using herpes virus
a technology of herpes simplex and chemotherapy, applied in the field of chemotherapy with herpes simplex virus, can solve the problems of unfavorable universal use of vaccines for herpes viruses, inability to fully explain the relationship between the role of hsv-specific virus genes and immune reactions, and the inability to fully understand the relationship between the role of hsv-specific virus genes and the severity of herpes virus infection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
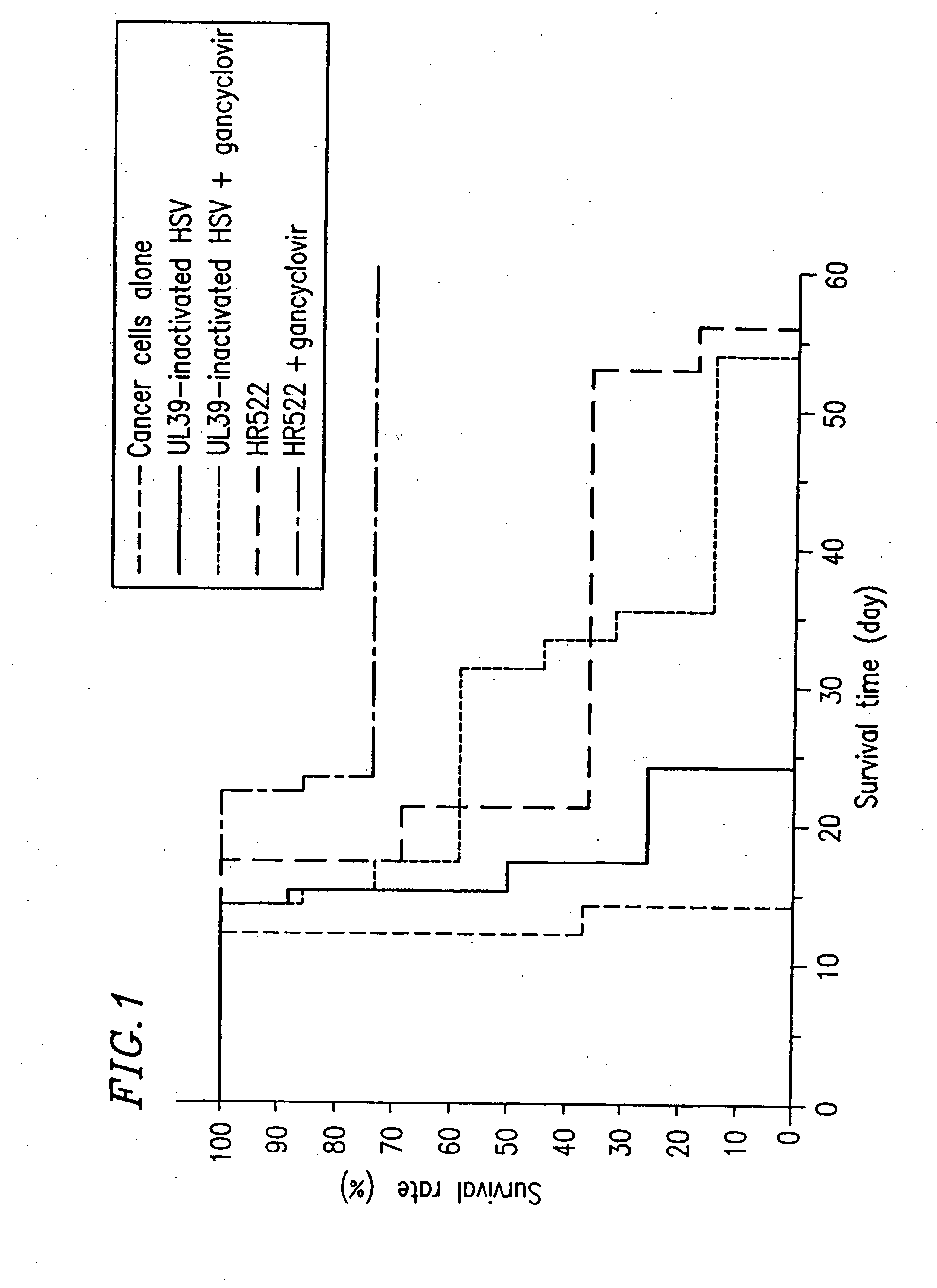
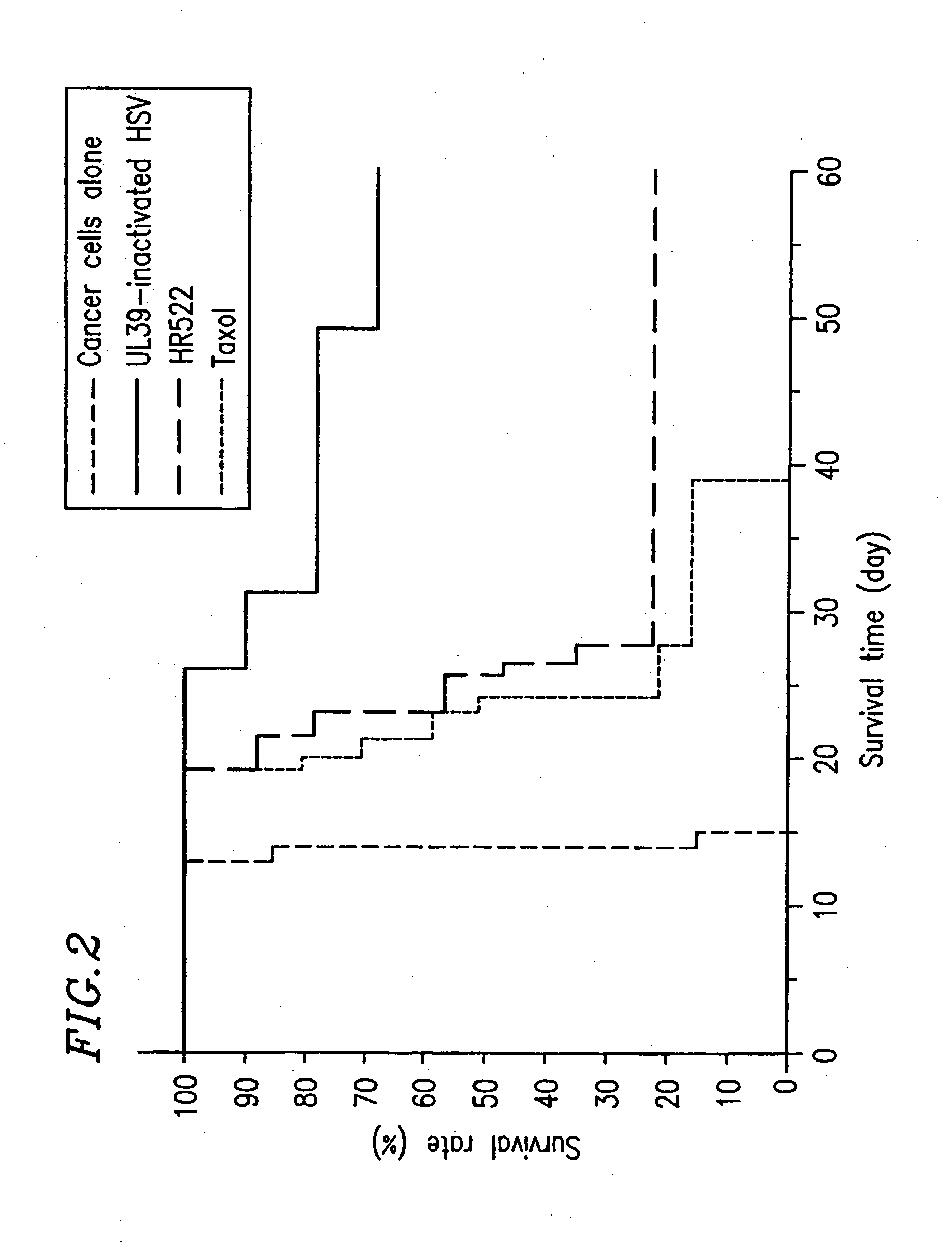
Preparation of Attenuated HSV (UL39-Inactivated HSV)
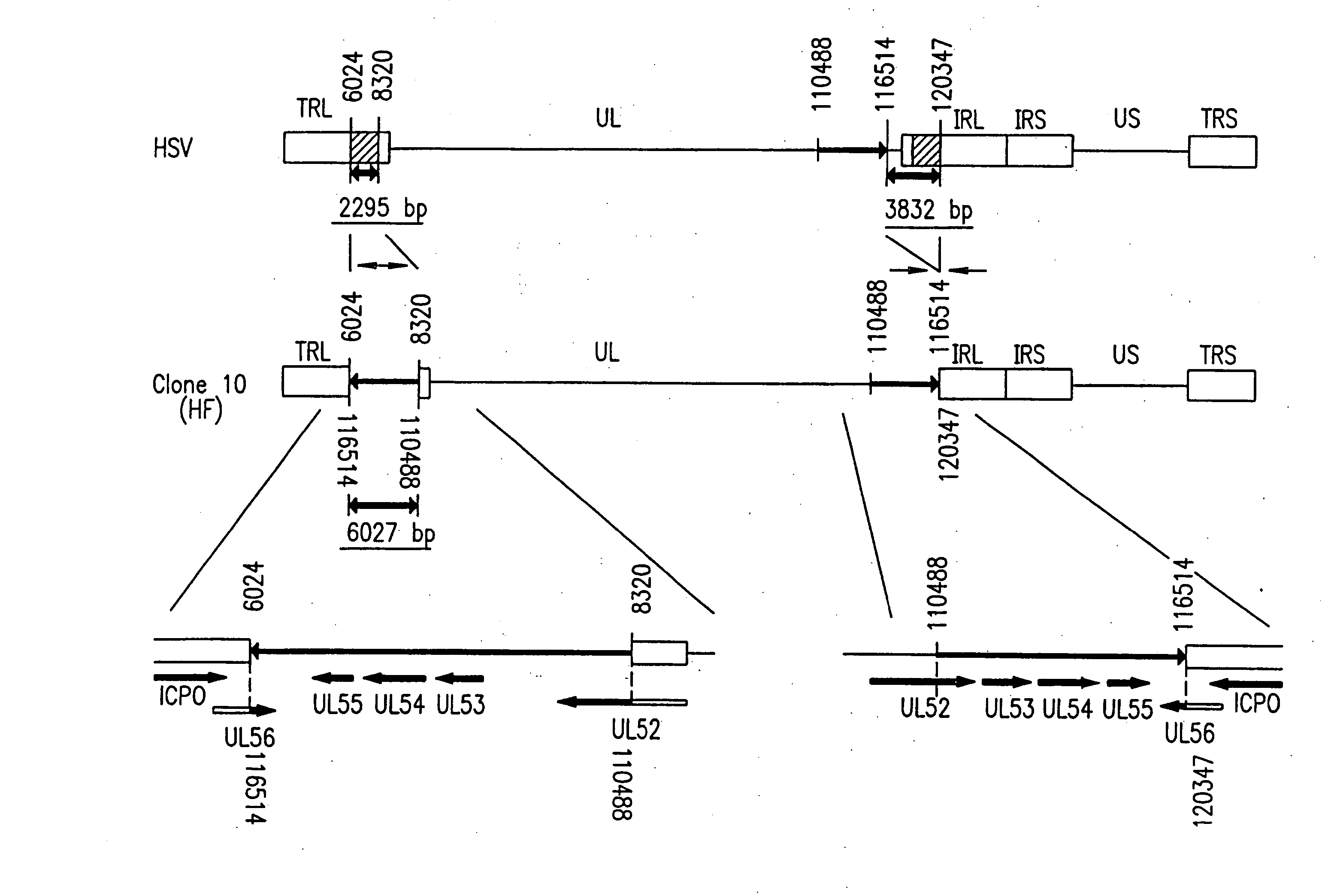
[0304] A DNA fragment of HSV-1 containing the UL39 gene was incorporated into plasmid pBluescript (manufactured by Stratagene) for cloning, so as to prepare DNA having a lacZ cassette interrupting the open reading frame of the UL39 gene. In this case, the lacZ cassette is a DNA fragment containing a promoter for the HSV UL39 at the 5′ end thereof and the lacZ gene of E. coli and the polyadenylation signal of SV40 downstream of the promoter. Infectious HSV DNA was prepared from cells infected with HSV. Cells were transfected with this DNA. After three days, the produced viruses were collected. A plaque which was stained blue in the presence of X-gal was collected. Further plaque cloning was conducted three times. The resultant viruses were multiplied in Vero cells and then stocked. The thus obtained mutant was subjected to Southern blot, PCR, Western blot, and the like, so that it was confirmed that UL39 was inactivated.
example 2
Preparation of Attenuated HSV (Hh) in which UL39 and UL56 Genes not Involved in DNA and Deoxyribonucleotide Metabolism are Inactivated
[0305] There is provided a strain HF derived from HSV-1 in which a region containing the UL56 gene is deficient (kindly provided by Dr. Shin Isomura, Department of Pediatrics, Nagoya University School of Medicine). The pathogenicity of this strain is significantly low when inoculated intraperitoneally in a mouse as compared to a wild type of the strain. Further, the strain causes cell fusion with infected cells. Vero cells were infected with the above-described UL39-deficient virus and the strain HF. Among the progeny viruses, viruses which cause cell fusion and produce blue plaque in the presence of X-gal were collected. After cloning the viruses, it was confirmed by PCR and Western blot that UL39 and UL56 are deficient in the viruses.
example 3
Preparation of Strain in which Carboxyesterase is Incorporated into UL39-Inactivated HSV
[0306] A human carboxyesterase gene having a promoter for the immediate early gene of human cytomegalovirus was inserted to a lacZ gene (open reading frame) so that DNA in which the lacZ gene was interrupted by the human carboxyesterase gene was prepared. Vero cells were transfected with this DNA and infectious DNA derived from UL39-inactivated virus which has lacZ. Produced viruses were collected. Viruses which produced colorless plaques in the presence of X-gal were collected, followed by plaque cloning. The resultant viruses were stored as a primary stock. Cell samples were infected with clones obtained as the primary stock. The cell samples were subjected to a fluorescent antibody technique using tagged antibodies. For positive clones, a secondary stock was prepared. The finally obtained clones were confirmed to induce carboxyesterase activity in infected cells.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com