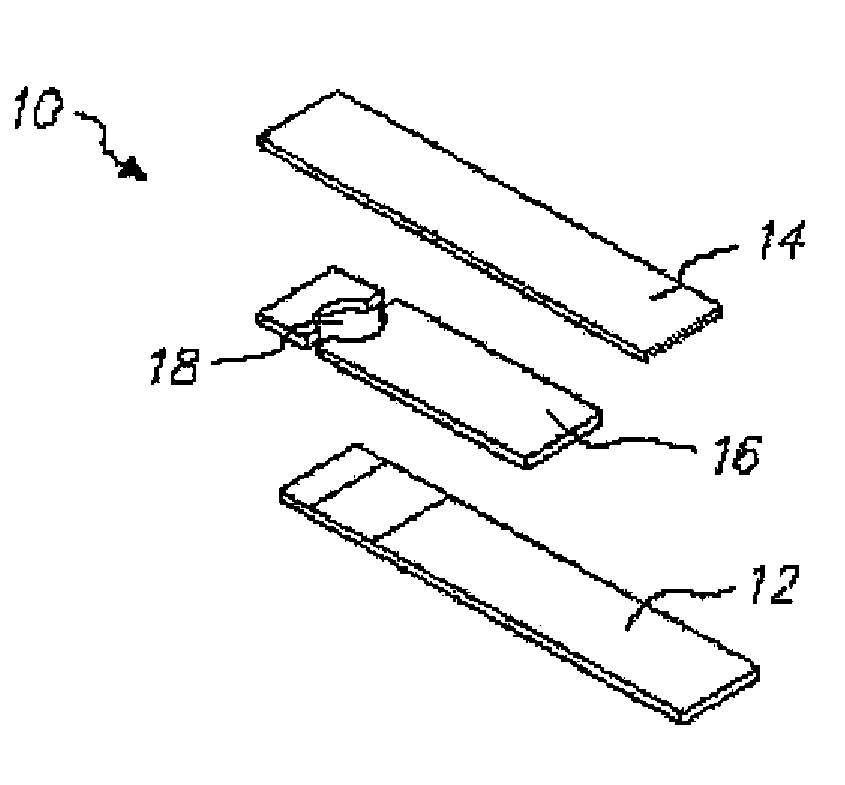
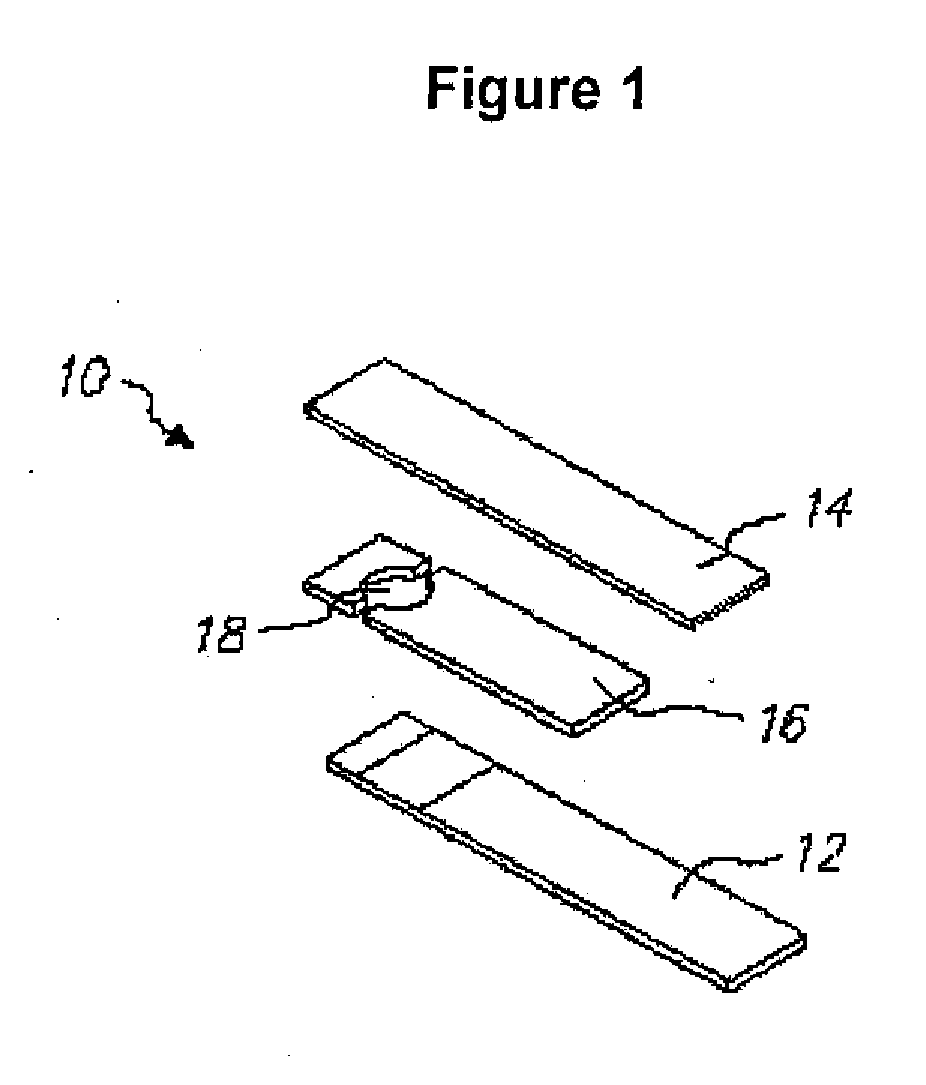
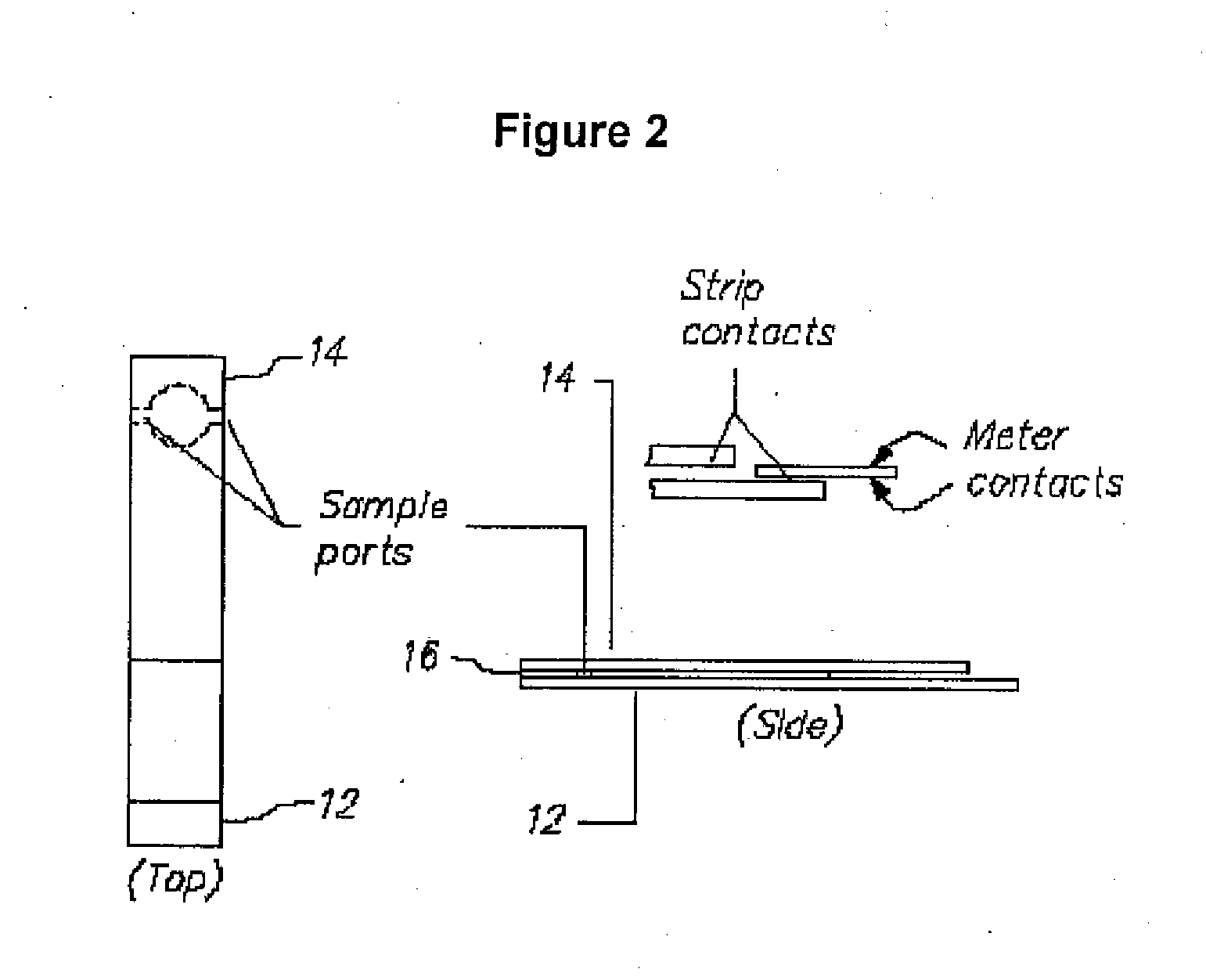
Mediator stabilized reagent compositions and methods for their use in electrochemical analyte detection assays
a technology of reagent composition and stabilized reagent, which is applied in the field of electrochemical analyte determination and electrochemical determination of blood analytes, can solve problems such as inaccurate results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Glucose Linearity Results Using Citrate, Malic, and Tartarate
[0068] Citric, malic, and tartaric buffer formulations were separately formulated at 100 mM concentration and pH 5.5. All 3 formulations also contained equivalent amounts of 0.1% anti-foam (RNA Equilibrator), 1 mM CaCl2, PQQ (2× mole ratio to GDH), 200 mM potassium ferricyanide, and 45 mg / mL GDH. Each formulation was ink-jetted onto a Pd substrate. The sensors were tested with blood containing glucose using chronoamperometry by applying a potential of −0.3 V for 10 sec, and then applying a potential of +0.3 V for 5 sec. Testing with blood showed good linearity with glucose for all cases (FIG. 3). Background for citrate and malic buffers were comparable while that of tartaric buffer was higher. In addition, tartaric buffer formed an insoluble salt with Ca2+ making it less desirable.
example 2
Glucose Linearity and Hematocrit Results Using Citrate and Mellitate
[0069] Citrate buffer was formulated at 400 mM and pH 5.5. Mellitate buffer was formulated at 160 mM and pH 6.4. Both formulations also contained equivalent amounts of 0.1% anti-foam (RNA Equilibrator), 1 mM CaCl2, PQQ (2× mole ratio to GDH), 200 mM potassium ferricyanide, and 32 mg / mL GDH. Blood testing was conducted using three hematocrit levels (20, 42, and 70%) and 4 glucose levels (FIGS. 4 and 5). The glucose response was not linear, but did increase with increasing glucose concentration. Hematocrit performance for citrate buffer was slightly better than mellitate. The results are provided in FIGS. 4 and 5.
example 3
Hematocrit and Stability Results Using Citrate and Citraconate
[0070] Citrate and citraconate buffers were separately formulated at 300 mM concentration and pH 6.5. Both formulations also contained equivalent amounts of 0.1% anti-foam (RNA Equilibrator), 4 mM CaCl2, PQQ (2× mole ratio to GDH), 800 mM potassium ferricyanide, and 46 mg / mL GDH. Formulations were deposited on Pd by means of inkjetting. Hematocrit performance for both citrate and citraconate buffers were similar (FIGS. 6 and 7). Accelerated aging at 56° C. for 14 days showed that citraconate had excellent background and performance stability as compared to citrate (Table 1). The bias represented in Table 1 is given as an absolute response difference at the 40 mg / dL glucose concentration, and as a percentage bias for glucose concentration greater than 100 mg / dL.
TABLE 1CITRACONICCITRATEBias to YSIBias to YSIGLUCOSEBias to YSIat Day 14Bias to YSIat Day 14LEVELat Day 0@56 C.at Day 0@56 C.406.985.384.57−7.062406.523.691.44−...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com