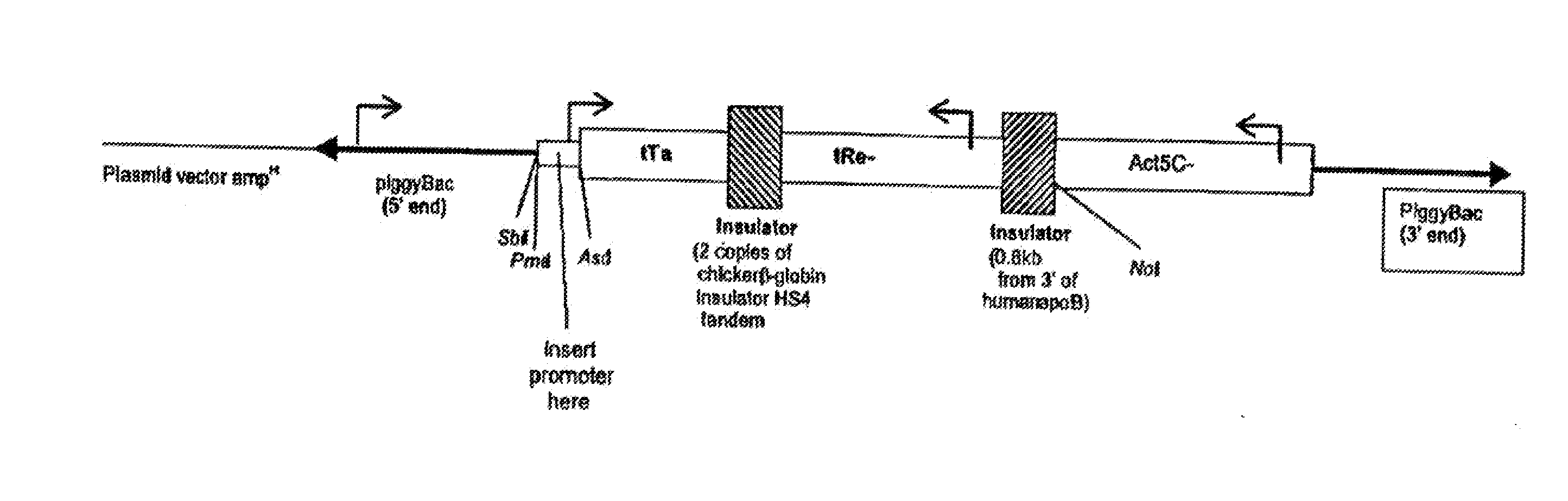
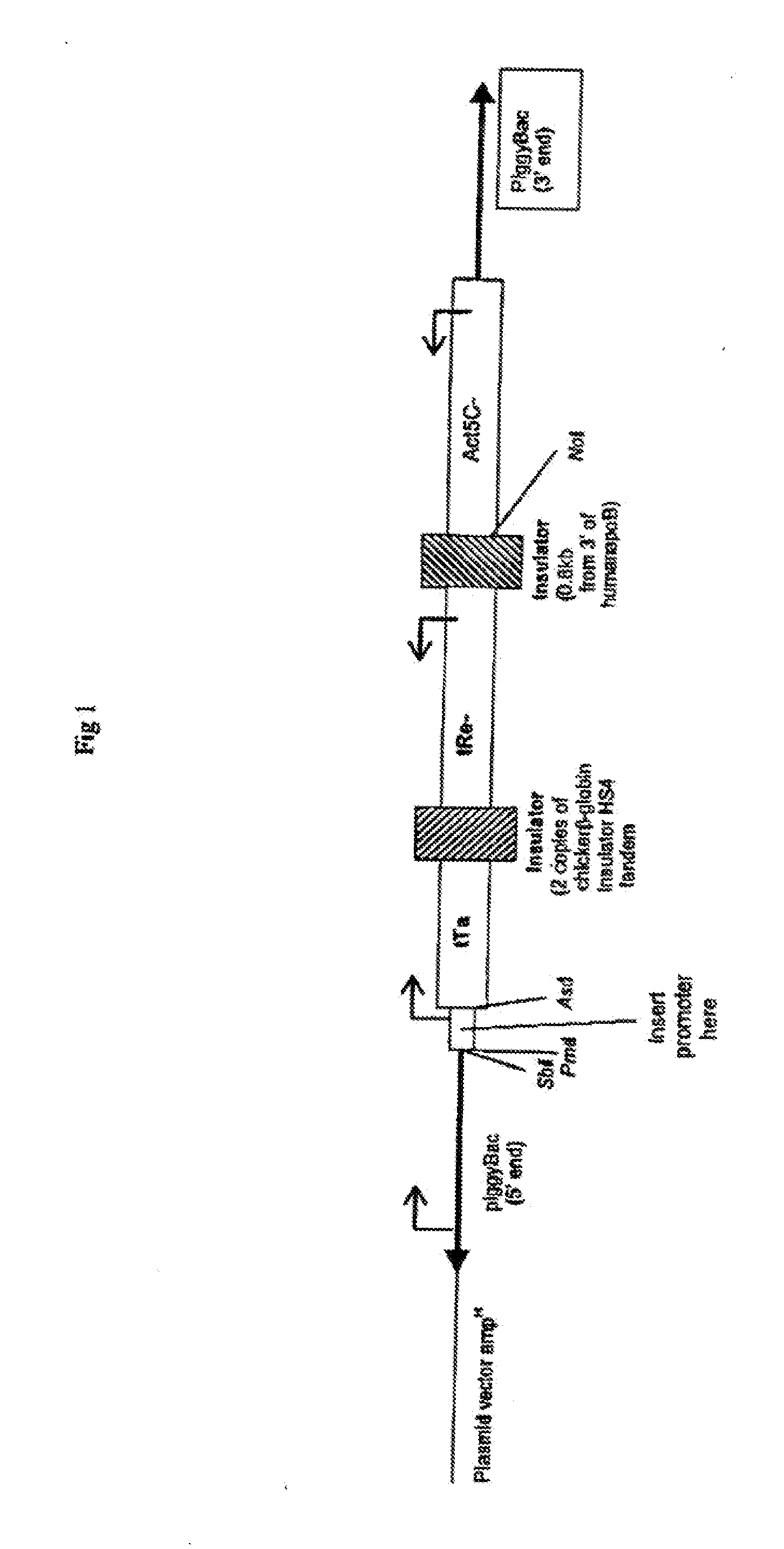
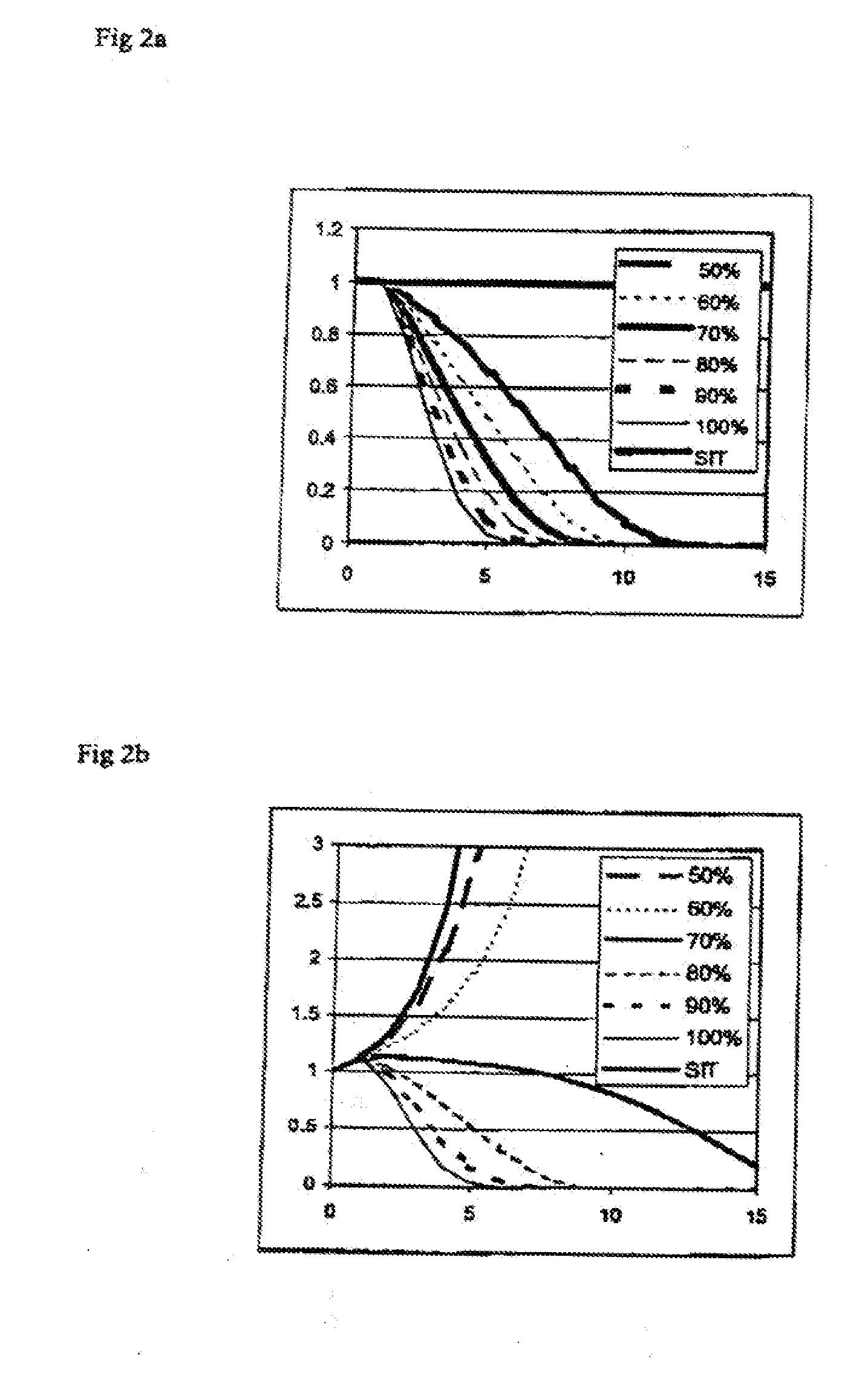
Biological Control
a technology of biological control and biological apparatus, applied in the field of biological control, can solve the problems of reducing the fitness of the resultant stock with respect, reducing the size of the next generation, and unable to reliably yield a truly single-sex population
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Single Chromosome Crosses
[0155] In “single chromosome crosses” at 25° C., ten to fifteen virgin females homozygous for the tTA construct and five to ten young males homozygous for the tRe construct were placed on food containing or lacking a tetracycline supplement. Their progeny were allowed to develop on this food.
[0156] Sxlpe
Sxlpe tTa(A,B,C,F) × tRe Ras64BV12(B,C)Tetracyclineconc. μg / mlFemaleTotalMaleTotal00A, 0B, 0C, 0F, 0, 0, 0, 0058, 47, 60, 51, 46, 60, 52, 544280.146, 49, 50, 51, 52, 50, 41, 4037956, 42, 72, 41, 56, 72, 61, 34434152, 40, 60, 0, 60, 72, 50, 5238650, 51, 55, 3, 63, 54, 57, 56389541, 55, 49, 52, 48, 47, 40, 5138336, 47, 42, 55, 36, 55, 52, 52375
[0157] Formal for data: the 8 numbers are the results from crosses using independent insertions of each element (to control for position effect). Here, 4 insertions of Sxlpe-tTA (A, B, C, and F) were used and two of tRE-Ras64Bv12 (B and C). The order of the data are: Sxlpe-tTA(A) females with tRe-Ras64Bv12(B) males, men...
example 2
Reporter Crosses
[0163] In “reporter crosses” at 25° C., females homozygous carrying an insertion of Sxlpe tTa on their X chromosome (Sxlpe tTa(A)) were crossed to males carrying various reporter constructs. As with “single chromosome crosses” ten to fifteen virgin females homozygous for the tTA construct and five to teas young males homozygous for the tRe construct were placed on food containing or lacking a tetracycline supplement. Their progeny were allowed to develop on this food.
lac-2
[0164] Embryos were stained for lacZ using a standard histochemical method.
Tetracyclineconc. μg / mlLacZ positiveTotalLacZ negativeTotal(Female) Sxlpc tTa(A) × tRe lacZ(III) (Male)060, 85, 99, 6030478, 89, 85, 933450.10, 0, 0, 00176, 174, 178, 18170910, 0, 0, 00188, 190, 181, 18073950, 0, 0, 00156, 151, 159, 185651(Male) Sxlpc tTa(A) × tRe lacZ(III) (Female)057, 82, 97, 4528161, 74, 59, 822760.10, 0, 0, 00131, 165, 132, 9051810, 0, 0, 00170, 161, 181, 19570750, 0, 0, 00126, 190, 190, 196702(Male...
example 3
Recombinant Chromosome Experiments
[0168] 40-45 young females and 20-25 young males raised at 25° C. upon food with the indicated tetracycline supplement were allowed to mate, then transferred to normal (tetracycline-free) food after 3-4 clays. These flies were transferred to fresh vials of normal food every day for 12 days, then removed on the 13th day. All the vials were incubated at 25° C. while the progeny developed. The numbers of male and female progeny emerging as adults in each vial were recorded.
Tetracycline Concentration
[0169] Sxlpe
Sxlpe-tTA, tRE-Ras64BV12 on the X chromosome.Tet.Day 1Day 2Day 3Day 4Day 5Day 6Day 7Conc. μg / mlMaleFemaleMaleFemaleMaleFemaleMaleFemaleMaleFemaleMaleFemaleMaleFemale 0.1103098089092010509501100 1128013701500136011108701000 51100111085090014409301380 2013101260133012009309901110 100139012701450110014901280940 5009511133121451137188011201260100014012133241198942921137112912000110359725941613812115212611451Tet.Day 8Day 9Day 10Day 11Day 12Tota...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com