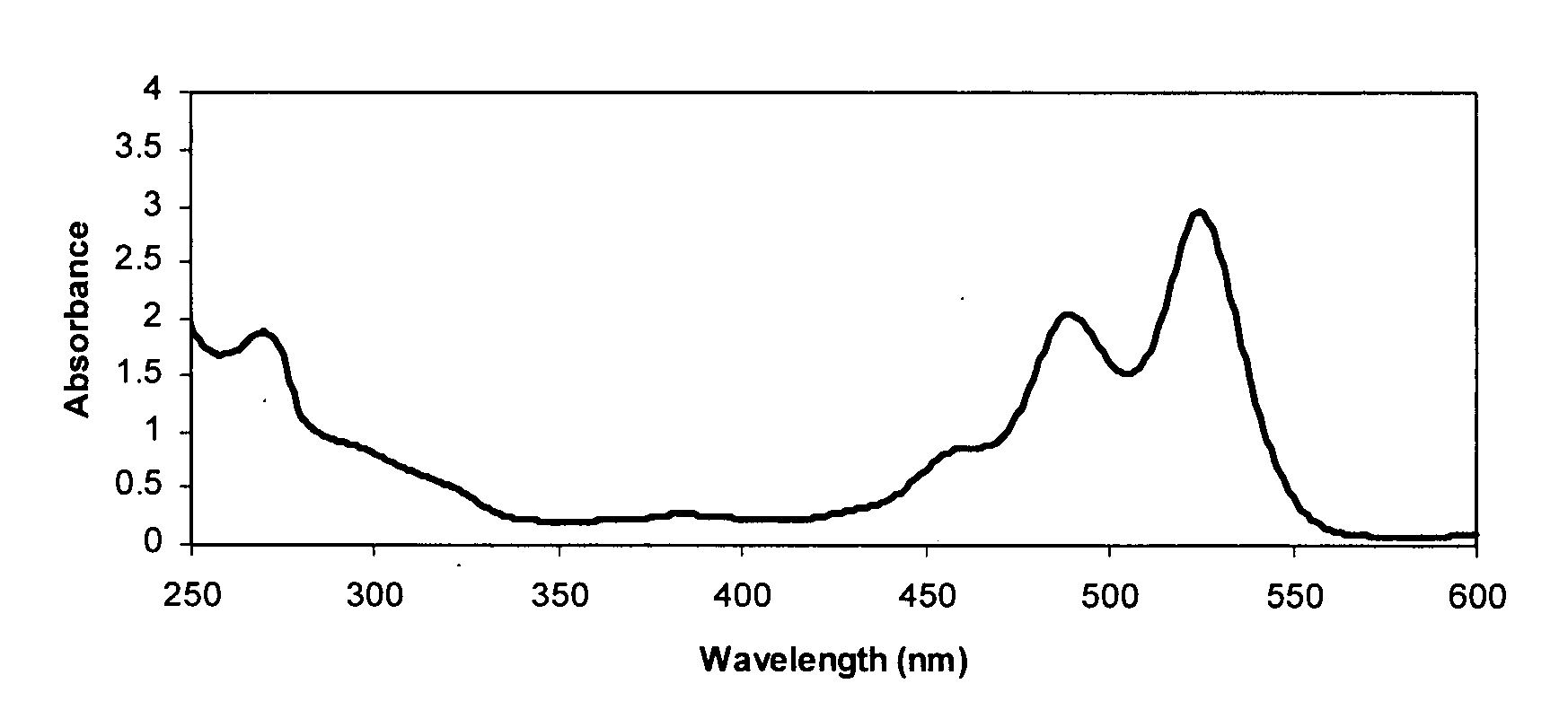
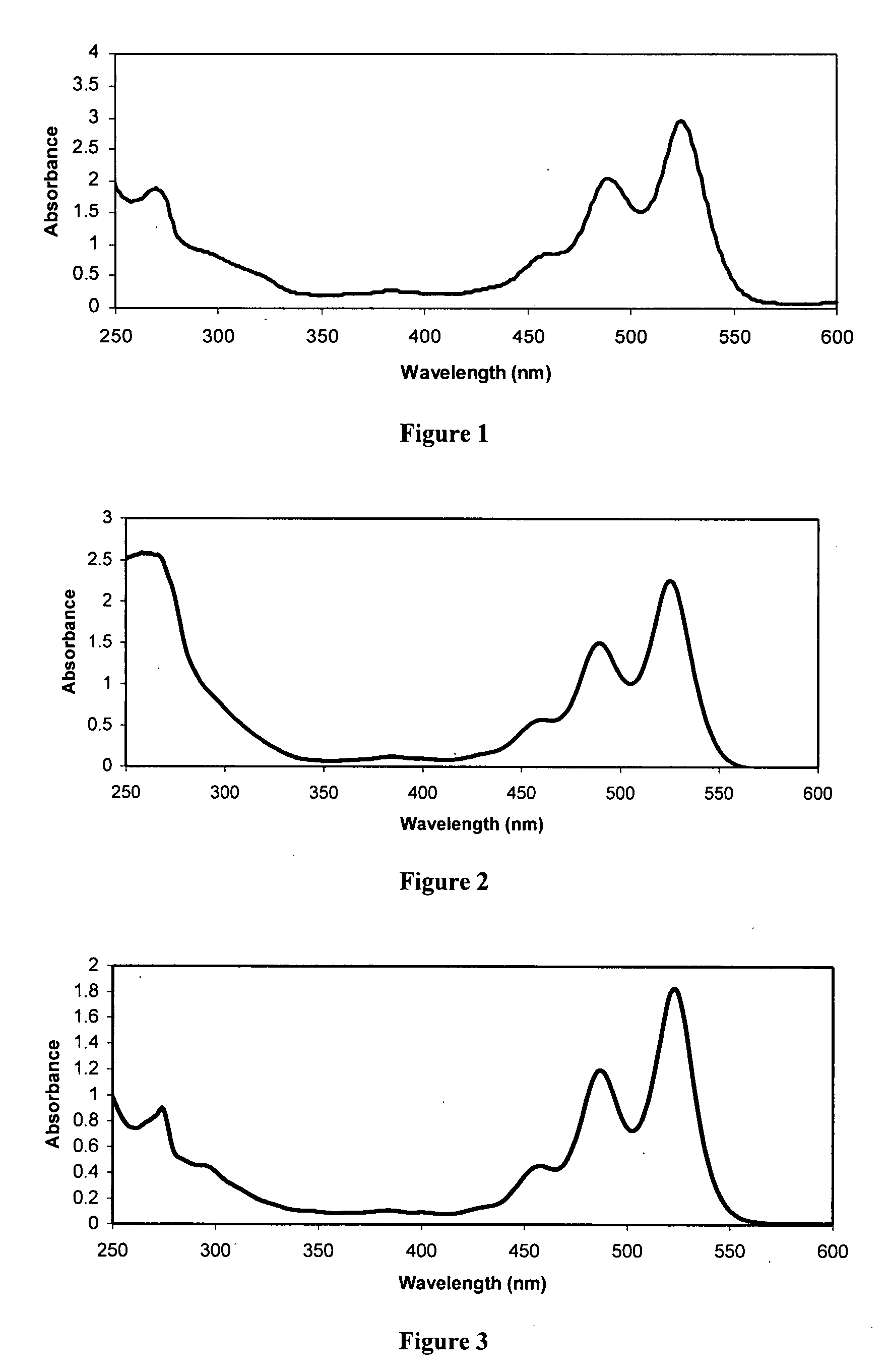
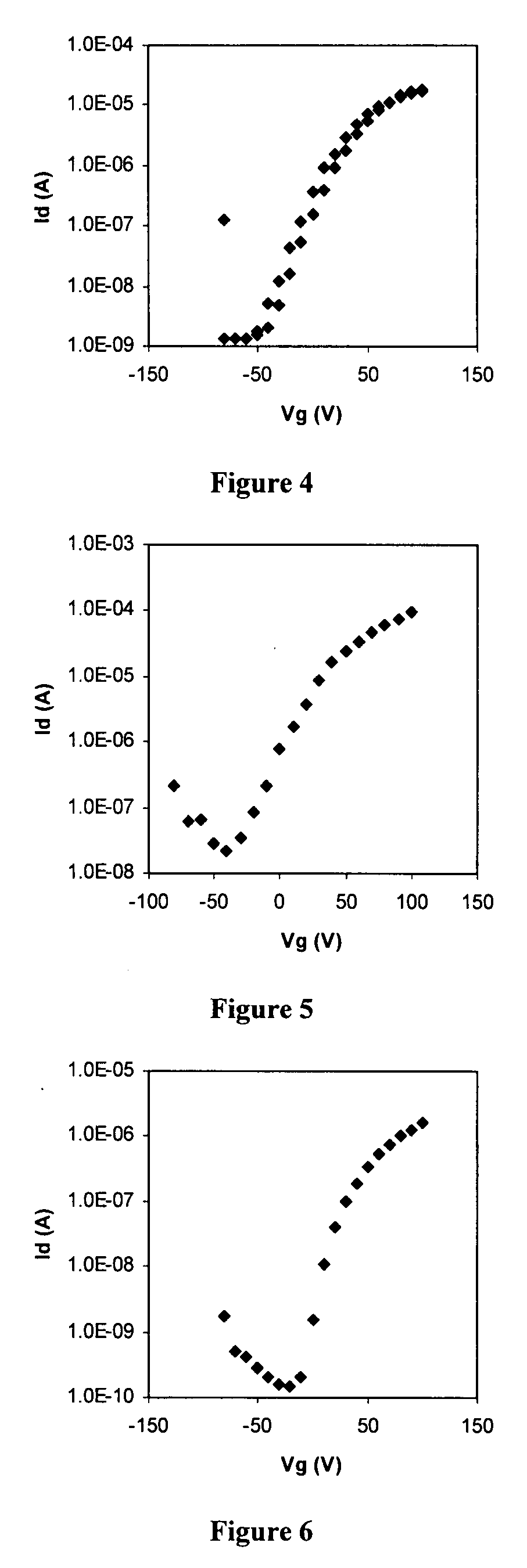
Diimide-based semiconductor materials and methods of preparing and using the same
a technology of semiconductor materials and diimide, which is applied in the manufacture of final products, naphthalimide/phthalimide dyes, anthracene dyes, etc., can solve the problems of poor air stability and limit the type of manufacturing process (e.g., printing deposition) that can be used with n-type semiconducting compounds, and achieves the effect of useful electrical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of N,N′-bis[(3S)-3,7-dimethyl-6-octenyl]-1,7-dicyanoperylene-3,4:9,10-bis(dicarboxiamide) (PDICitr-CN2)
Part A: Preparation of N,N′-bis[(3S)-3,7-dimethyl-6-octenyl]-1,7-dibromoperylene-3,4:9,10-bis(dicarboxiamide) (PDICitr-Br2
[0112]Citronellylamine (5.00 g, 31.8 mmol) was added to a suspension of 1,7-dibromoperylene-3,4:9,10-dianhydride (PDABr2) (4.37 g, 7.95 mmol) in propionic acid (50 mL). The reaction mixture was heated under reflux for 12 hours. After cooling to room temperature, the resulting solid was collected by filtration, washed with propionic acid and several times with methanol (MeOH), and finally dried overnight. The crude product (4.71 g) was purified by chromatography on silica gel (dichloromethane, CH2Cl2) to give N,N′-bis[(3S)-3,7-dimethyl-6-octenyl]-1,7-dibromoperylene-3,4:9,10-bis(dicarboxiamide) as a red solid (0.70 g, 0.85 mmol, 10.7% yield).
[0113]M.p.=270-272° C. (DMF); 1H NMR (CHCl3, 400 MHz): δ 9.50 (d, 2H, J=7.5 Hz), 8.94 (s, 2H), 8.72 (d, 2H, J=...
example 2
Preparation of N,N′-bis(4-n-hexylphenyl)-1,7-dicyanoperylene-3,4:9,10-bis(dicarboxiamide) (PDIPh6-CN2)
Part A: Preparation of N,N′-bis(4-n-hexylphenyl)-1,7-dibromoperylene-3,4:9,10-bis(dicarboxiamide) (PDIPh6-Br2)
[0116]4-n-Hexylaniline (3.39 g, 19.12 mmol) was added to a suspension of 1,7-dibromoperylene-3,4:9,10-dianhydride (4.00 g, 4.78 mmol) in propionic acid (40 mL). The reaction mixture was heated under reflux for 12 hours. After cooling to room temperature, the resulting solid was collected by filtration, washed with propionic acid and several times with MeOH, and finally dried overnight. The crude product (4.38 g) was purified by chromatography on silica gel (chloroform:acetone—50:50) to give N,N′-bis(4-n-hexylphenyl)-1,7-dibromoperylene-3,4:9,10-bis(dicarboxiamide) as a red solid (1.49 g, 1.72 mmol, 36.0% yield).
[0117]M.p. >250° C. (DMF); 1H NMR (CHCl3, 400 MHz): δ 9.54 (d, 2H, J=8.0 Hz), 8.96 (s, 2H), 8.76 (d, 2H, J=8.0 Hz), 7.40 (d, 4H, J=8.0 Hz), 7.24 (d, 4H, J=8.0 Hz), 2....
example 3
Preparation of N,N′-bis(4-n-dodecylphenyl)-1,7-dicyanoperylene-3,4:9,10-bis(dicarboxiamide) (PDIPh12-CN2)
Part A: Preparation of N,N′-bis(4-n-dodecylphenyl)-1,7-dibromoperylene-3,4:9,10-bis(dicarboxiamide) (PDIPh12-Br2)
[0120]4-Dodecylaniline (5.00 g, 19.12 mmol) was added to a suspension of 1,7-dibromoperylene-3,4:9,10-dianhydride (2.63 g, 4.78 mmol) in propionic acid (52.5 mL). The reaction mixture was heated under reflux for 12 hours. After cooling to room temperature, the resulting solid was collected by filtration, washed with propionic acid and several times with MeOH, and finally dried overnight. The crude product (3.25 g) was extracted using a Soxhlet extractor with chloroform (CHCl3, 500 mL) over 2 days and the resulting solid was purified by chromatography on silica gel (CH2Cl2) to afford N,N′-bis(4-n-dodecylphenyl)-1,7-dibromoperylene-3,4:9,10-bis(dicarboxiamide) as a red solid (1.20 g, 1.11 mmol, 24.2% yield).
[0121]M.p. >300° C. (DMF); 1H NMR (CHCl3, 500 MHz): δ 9.58 (d, 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Area | aaaaa | aaaaa |
| Area | aaaaa | aaaaa |
| Area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com