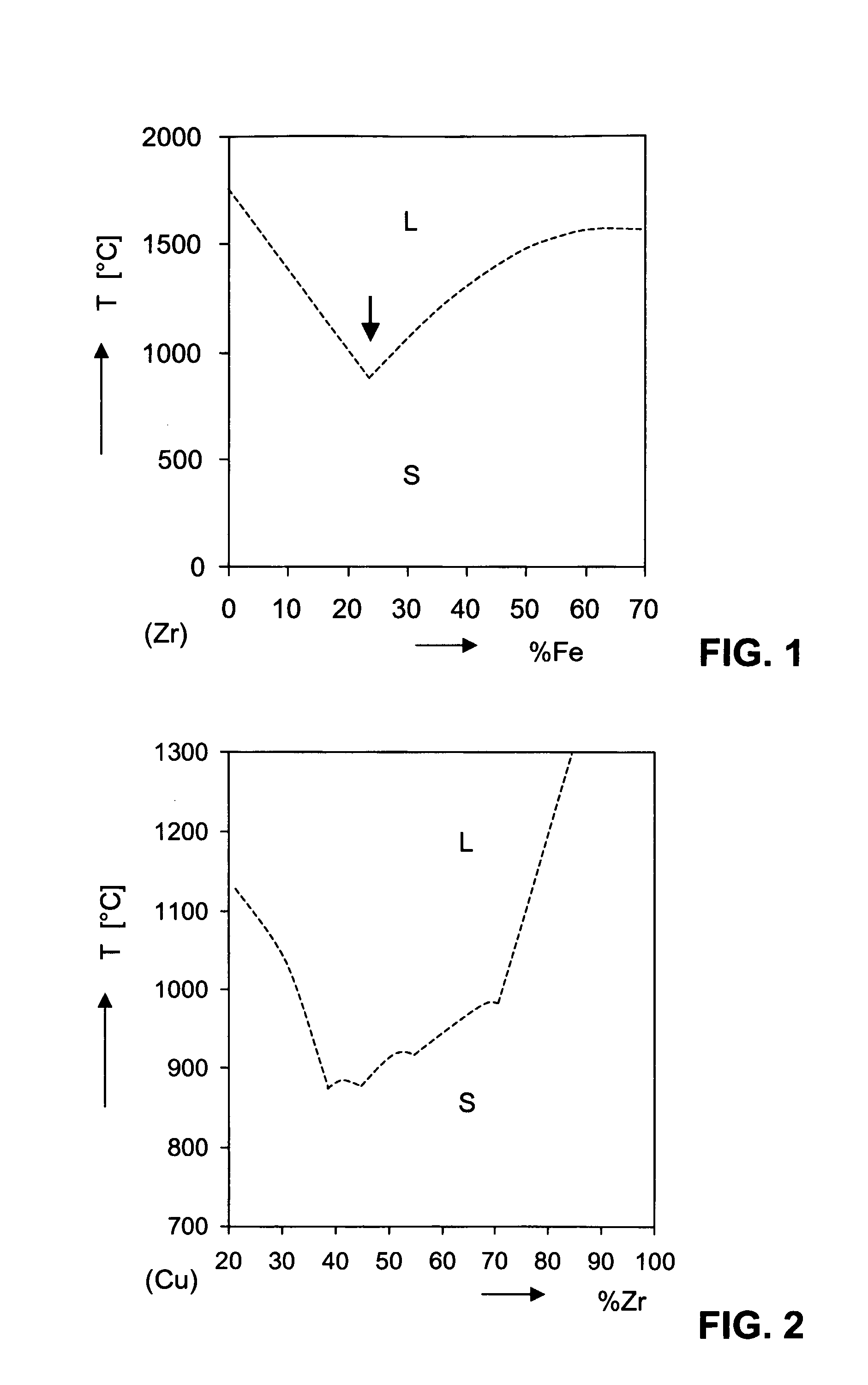
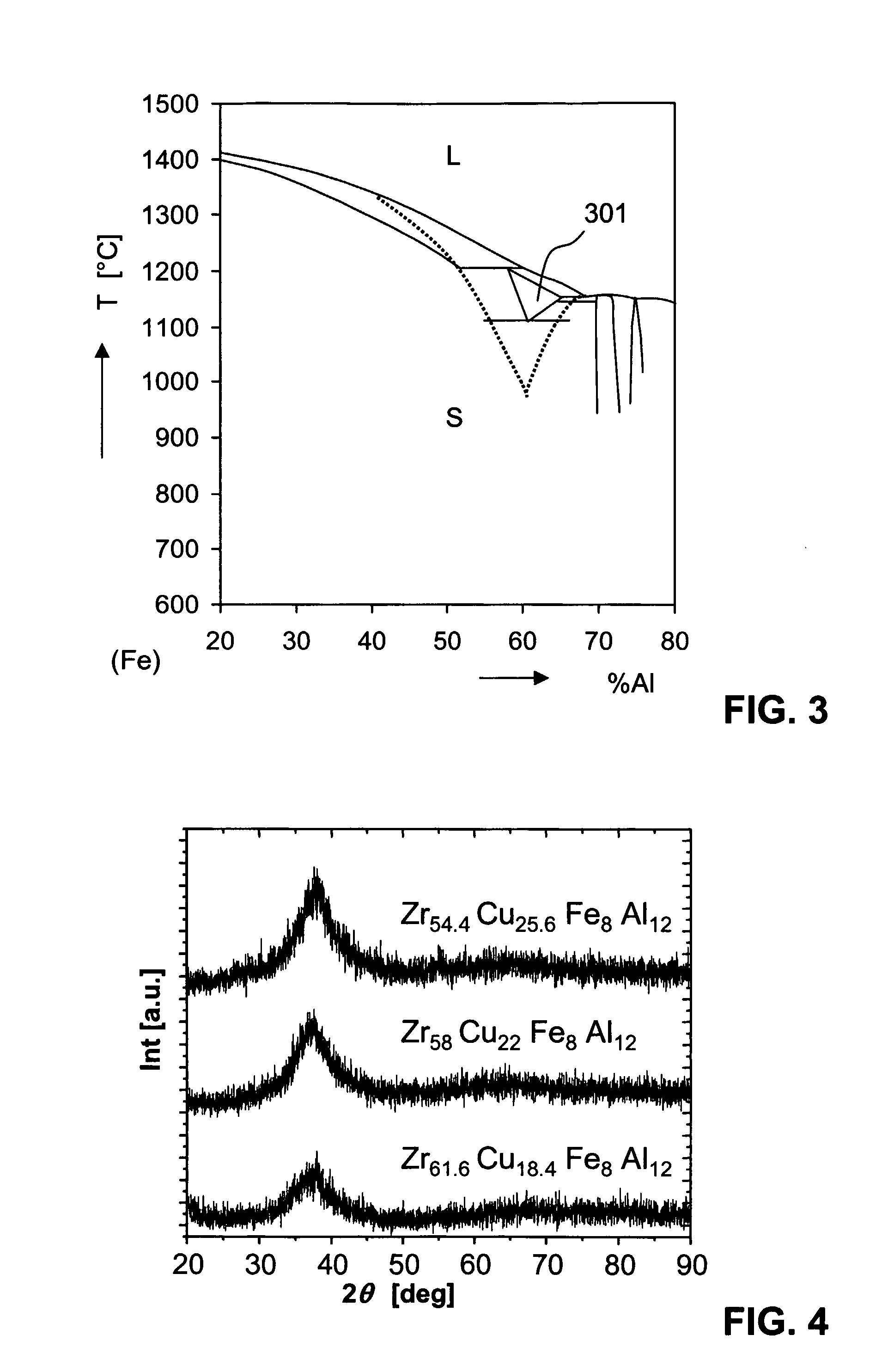
Amorphous Alloys on the Base of Zr and their Use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation and Characterization of Amorphous (ZrxCu100−x)80(Fe40Al60)20 Samples
[0098]Several Zr-based Ni-free alloys with composition (ZrxCu100−x)80(Fe40Al60)20 were prepared, where x=60, 62, 64, 66, 68, 72.5, 77, 79, 81, 83 and 85. Ingots were prepared by arc melting the constituents (purity >99.9%) in a titanium-gettered argon atmosphere (99.9999% purity). Using an induction-heating coil, the ingots were remelted in a quartz tube (vacuum ≈10−5 mbar) and injection cast into a copper mold with high-purity argon. Samples were cast into plates with a thickness of 0.5 mm, width of 5 mm and length of 10 mm. To determine the critical casting thickness, some samples were additionally or alternatively cast into various rod- and cone-like shapes with diameters ranging up to 10 mm. Furthermore, several samples were made with a thickness of 1 mm and cross section 1 cm×4 cm. The samples were then, where appropriate, cut into various pieces of length 1 cm and investigated by X-ray diffraction ...
example 2
Preparation of Mixed-Phase Samples
[0113]Samples with a mixed-phase structure were prepared as follows: Fully amorphous samples of Zr58Cu22Fe8Al12 were prepared as in Example 1. The samples were subjected to heat treatment (annealing) at various temperatures for 12 hours. XRD patterns and DTA scans were recorded for the heat-treated samples. FIG. 15 shows XRD patterns of the samples in the as-prepared state (bottom trace) and after annealing. The XRD patterns show typical amorphous structures up to an annealing temperature of 683 K. At higher annealing temperatures, however, clear Bragg peaks arising from an icosahedral phase (I.P.) can be observed. At still higher temperatures, peaks which are typical for a Zr2Fe structure are observed. FIG. 16 shows the XRD pattern of the sample annealed at 708 K for 12 hours in more detail. The indexing indicates the presence of an icosahedral phase with a lattice constant of 0.476 nm. FIG. 17 shows DTA scans of the same samples as in FIG. 15, whi...
example 3
Variations of Composition
[0116]Samples in a widely varying range of compositions were prepared and investigated. The compositions of the following Tables proved to be at least partially amorphous when cast to a plate with thickness of 1 mm (Table 4), 0.5 mm (table 5), or 0.2 mm (Table 6):
TABLE 4Alloys having a partially or fully amorphous structure when cast to athickness of 1 mm.(Zr95Ti5)72Cu13Fe13Al2Zr72Cu12Fe12Al4Zr70Cu13Fe13Al3Sn1Zr70Cu13Fe13Al4Zr70Cu13Fe13Al2Cr2Zr72Cu11Fe11Al6Zr70Cu13Fe13Al2Nb2Zr72Cu11.5Fe11Al5.5Zr70Cu13Fe13Al2Zn2Zr73Cu11Fe11Al5(Zr72Cu13Fe13Al2)98Mo2Zr71Cu11Fe11Al7(Zr72Cu13Fe13Al2)98P2Zr69Cu11Fe11Al9(Zr95Hf5)72Cu13Fe13Al2Zr70Cu10.5Fe10.5Al9Zr70Cu11Fe11Al8Zr70Cu10Fe11Al9Zr71Cu11Fe10Al8Zr70Cu11Fe10Al9(Zr74Cu13Fe13)90Al10Zr69Cu10Fe10Al11Zr72Cu13Fe13Al2Zr69Cu10Fe11Al10(Zr74Cu13Fe13)98Al2Zr70Cu13Fe13Al2Sn2Zr73Cu13Fe13Al1Zr72Cu13Fe13Sn2Zr72Cu13Fe13Al2(Zr74Cu13Fe13)98Sn2Zr71Cu13Fe13Al3
TABLE 5Alloys with a partially or fully amorphous structure when cast to athickness ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cooling rate | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com