Antigen binding molecules that bind EGFR, vectors encoding same, and uses thereof
a technology of egfr and binding molecules, applied in the field of antigen binding molecules, can solve the problems of poor prognosis of patients, induction of immune responses, and correlation or association of overexpression of egfr, and achieve the effect of enhancing the affinity and effector function of fc receptors and enhancing the efficacy of these abms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0288]High Homology Acceptor Approach
[0289]The high homology antibody acceptor framework search was performed by aligning the rat ICR62 protein sequence to a collection of human germ-line sequences and choosing that human sequence that showed the highest sequence identity, while conserving all canonical residues on a functional level. Here, the sequence 1-e from the VH1 family within the VBase database was chosen as the heavy chain framework acceptor sequence, and the A30 sequence from the VK1 family of the VBase database was chosen to be the framework acceptor for the light chain. On these two acceptor frameworks the three complementarity determining regions (CDRs) and / or specificity-determining residues of those CDRs of the rat ICR62 heavy and light variable domains were grafted. Since the framework 4 region is not part of the variable region of the germ line gene, the alignment for that position was performed individually. The JH6 region was chosen for the he...
example 2
Results and Discussion
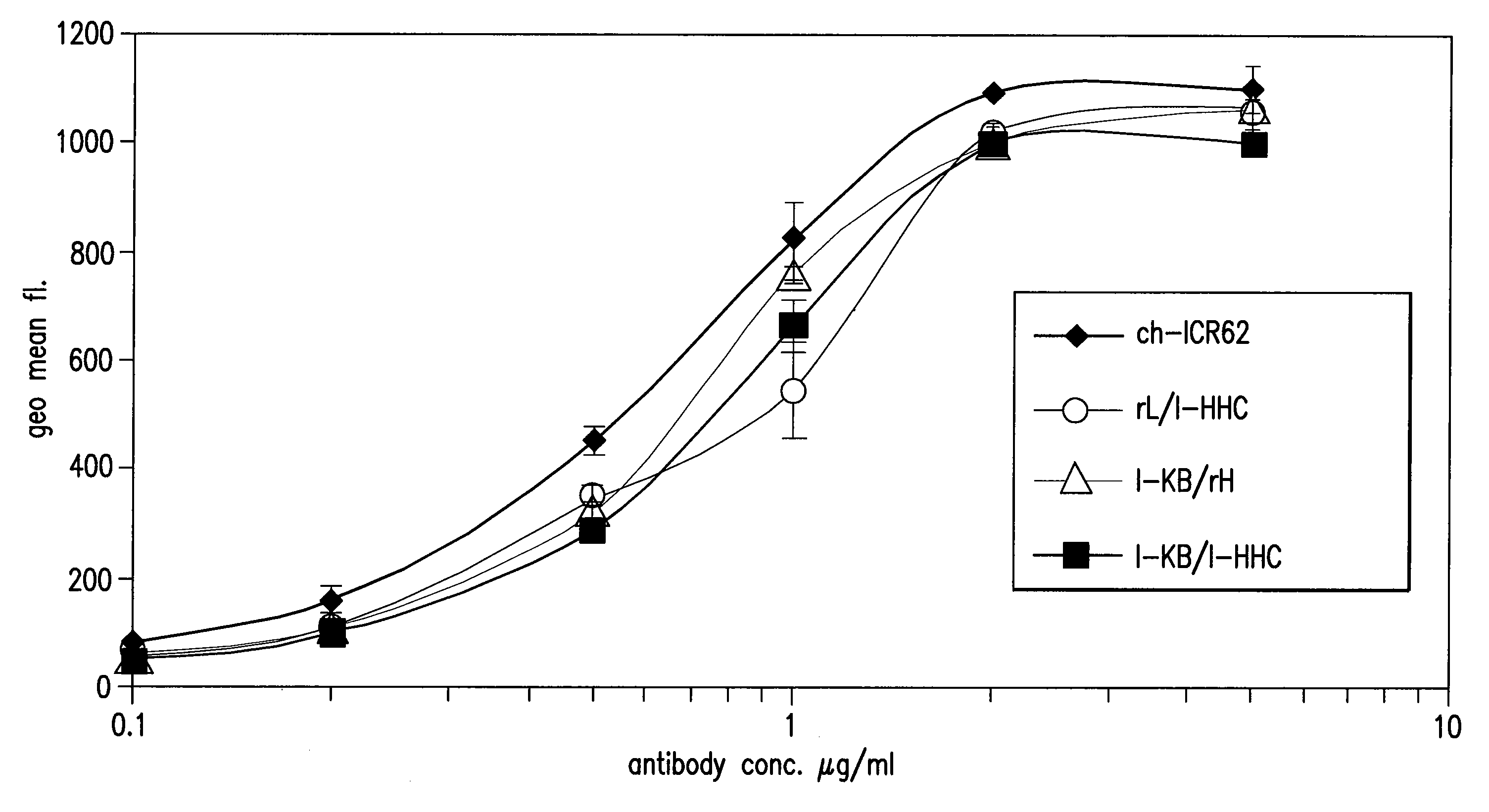
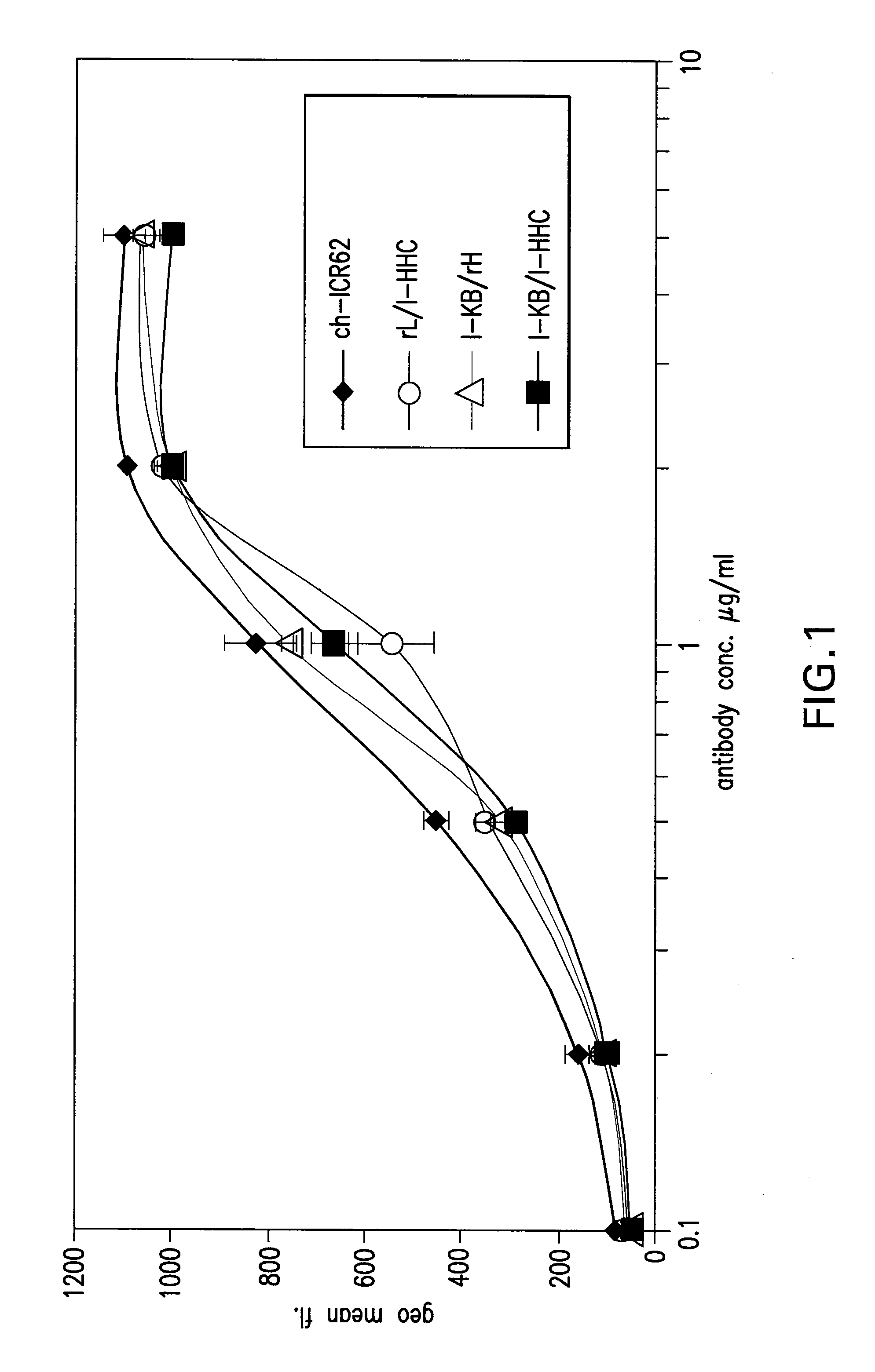
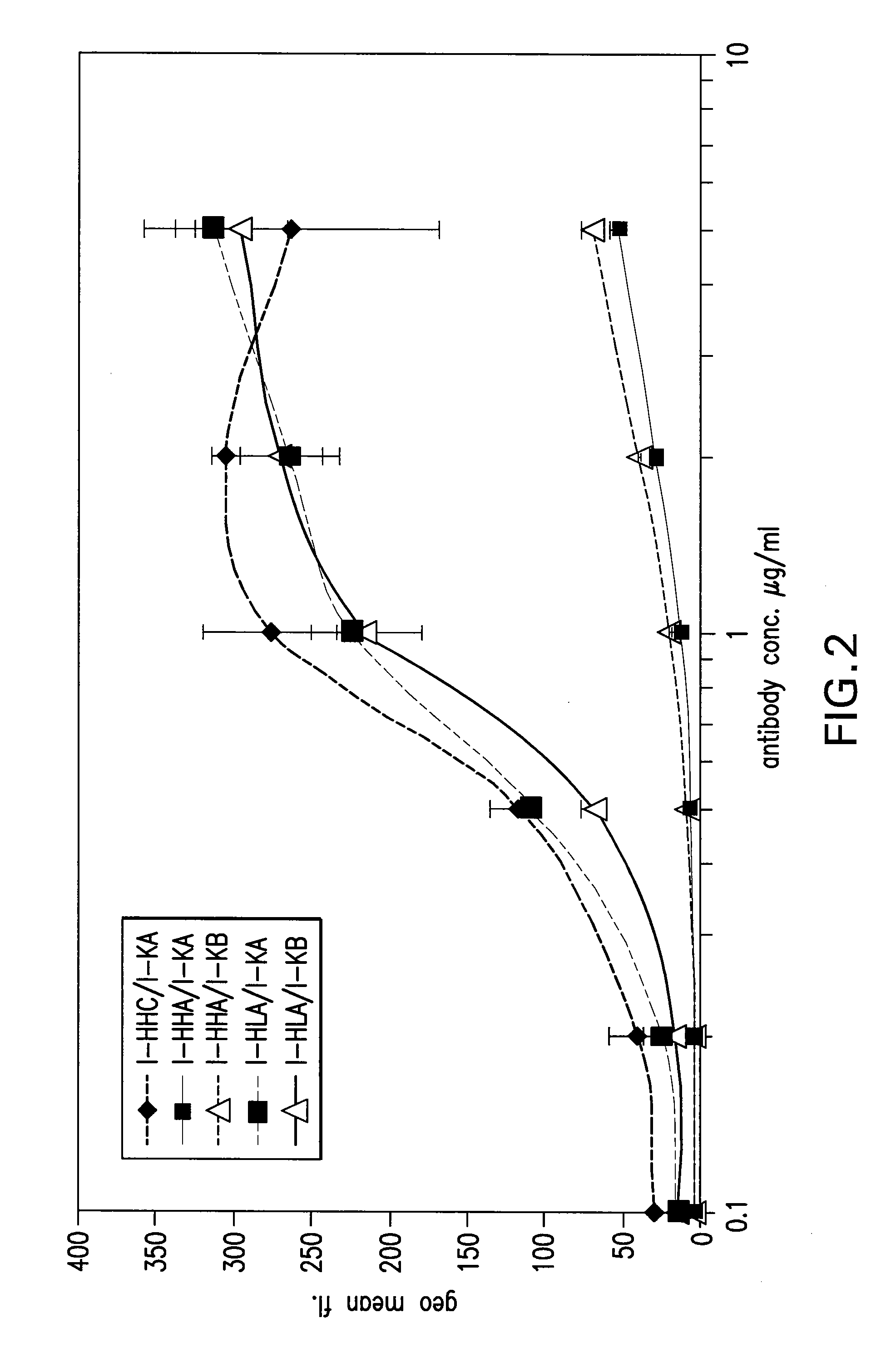
[0315]Comparison of the binding to human EGF-receptor of antibody variants I-HHA, I-HHB, I-HHC, I-HLA, I-HLB, I-HLC, I-HLA1, I-HLA2, I-HLA3, I-HLA4, I-HLA5, I-HLA6, I-HLA7, I-HLA8, I-HLA-9, I-HHD, I-HHE, I-HHF, and I-HHG, either complexed with the chimeric ICR62 light chain or with the humanized ICR62 light chains (1-KA, I-KB, or I-KC) and the parental, chimeric antibody ch-ICR62 shows that all antibodies have within one log unit similar EC50 values. Only the I-HHA has strongly diminished binding activity (see FIG. 2). FIG. 1 shows the functional activity of the individual chimeric ICR62 (ch-ICR62) polypeptide chains when combined with the humanized constructs I-HHC and I-KB, respectively. In this experiment, either the light chain, the heavy chain or both chains simultaneously of the ch-ICR62 were replaced by the above mentioned humanized constructs. This shows that the VH / VL interface formation seems to work as well in the rodent antibody as well as in the hu...
example 3
Preliminary Toxicity Study by Intravenous (Bolus) Administration to Cynomolgus Monkeys
Bioanalytical Analysis
Introduction
[0338]Glyco-Engineered Anti-EGFR Assay
[0339]This Bioanalytical Analysis describes the measurement of anti-EGFR in samples originating from cynomolgus monkeys following intravenous (bolus) administration of anti-EGFR (recombinant, glycoengineered anti-EGFR antibody produced from transfected mammalian cells in culture with antibody expression vectors harboring the heavy chain I-HHB and the light chain I-KC genes as described above, and purified as described above) as described in the protocol set forth herein below. A total of 78 monkey serum samples were stored frozen at about −20° C. until use.
[0340]The Bioanalytical methods used for the determination of anti-EGFR used an ELISA method to measure serum concentrations of anti-EGFR. Acceptance criteria were set at ±20% (±25% low QC) for precision and inaccuracy.
Materials and Methods
[0341]Objective: The objective of th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com