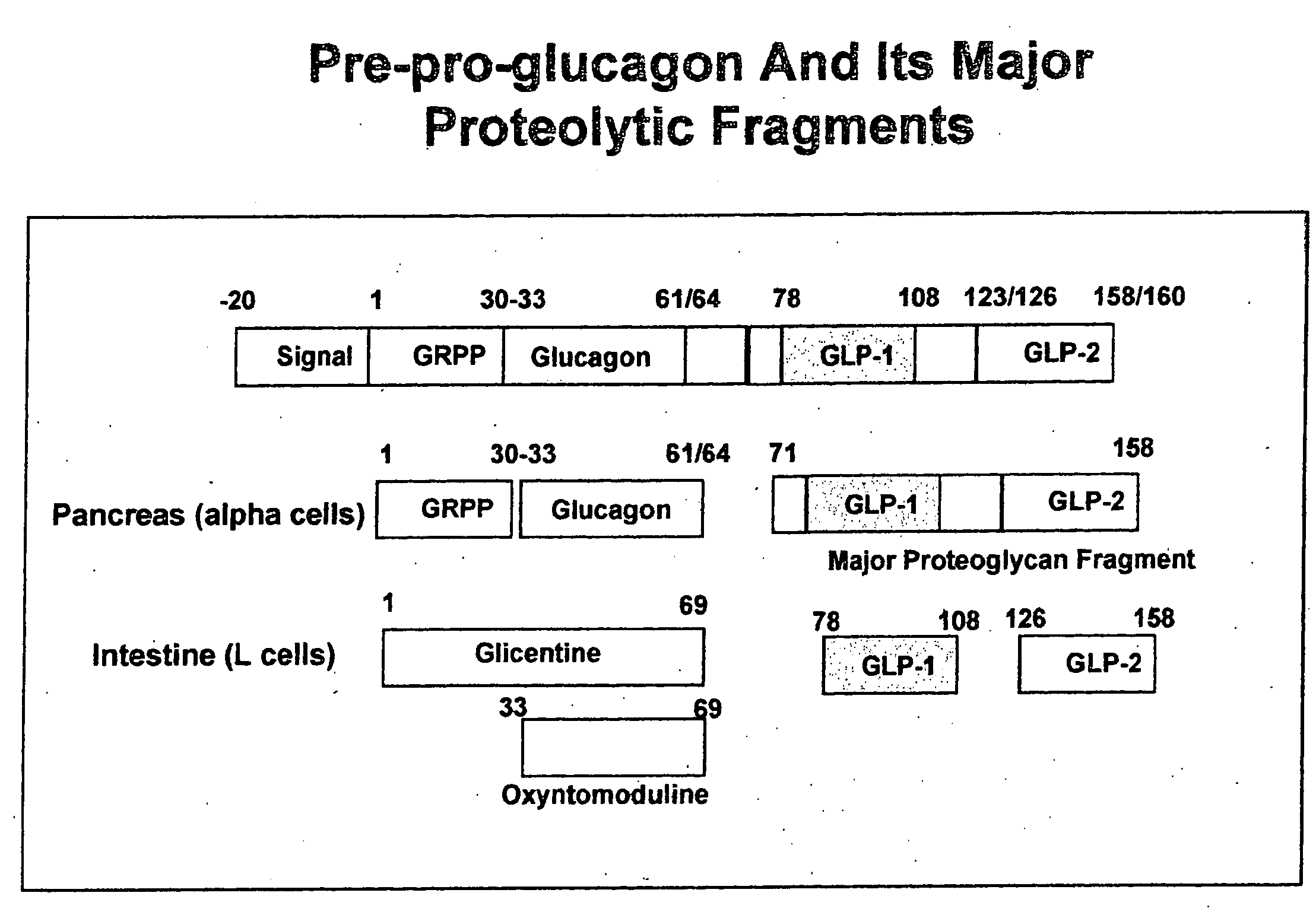
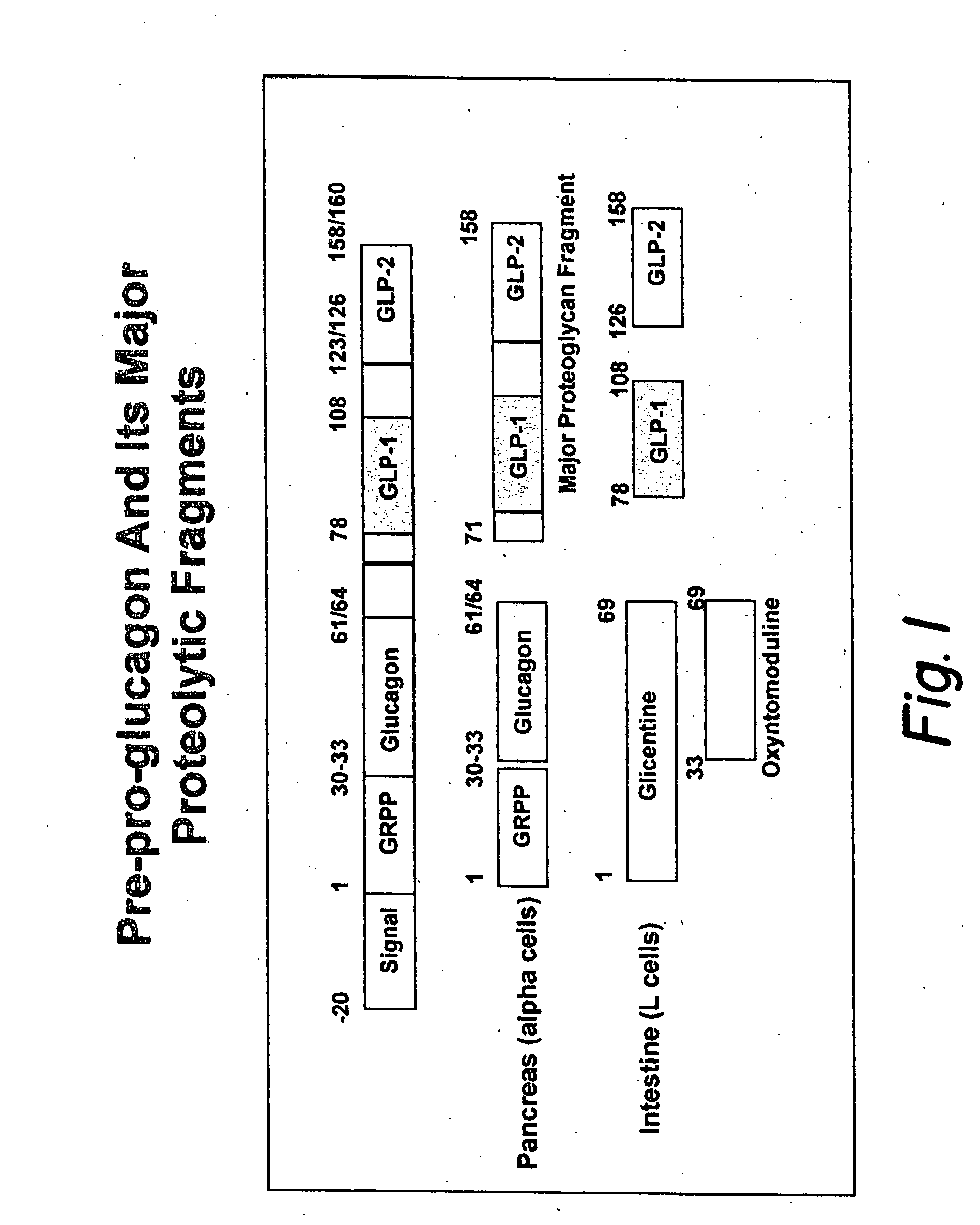
Modified nucleotide sequence encoding glucagon-like peptide-1 (glp-1), nucleic acid construct comprising same for production of glucagon-like peptide-1 (glp-1), human cells comprising said construct and insulin-producing constructs, and methods of use thereof
a nucleotide sequence and glucagon-like peptide technology, which is applied in the field of modified nucleotide sequence encoding glucagon-like peptide-1 (glp-1), can solve the problems of short half life, inability to rapidly degrade, and limited potential as a new therapeutic agent, so as to reduce increase the glucose blood level, and reduce the weight of the subj
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
GLP-1 Constructs
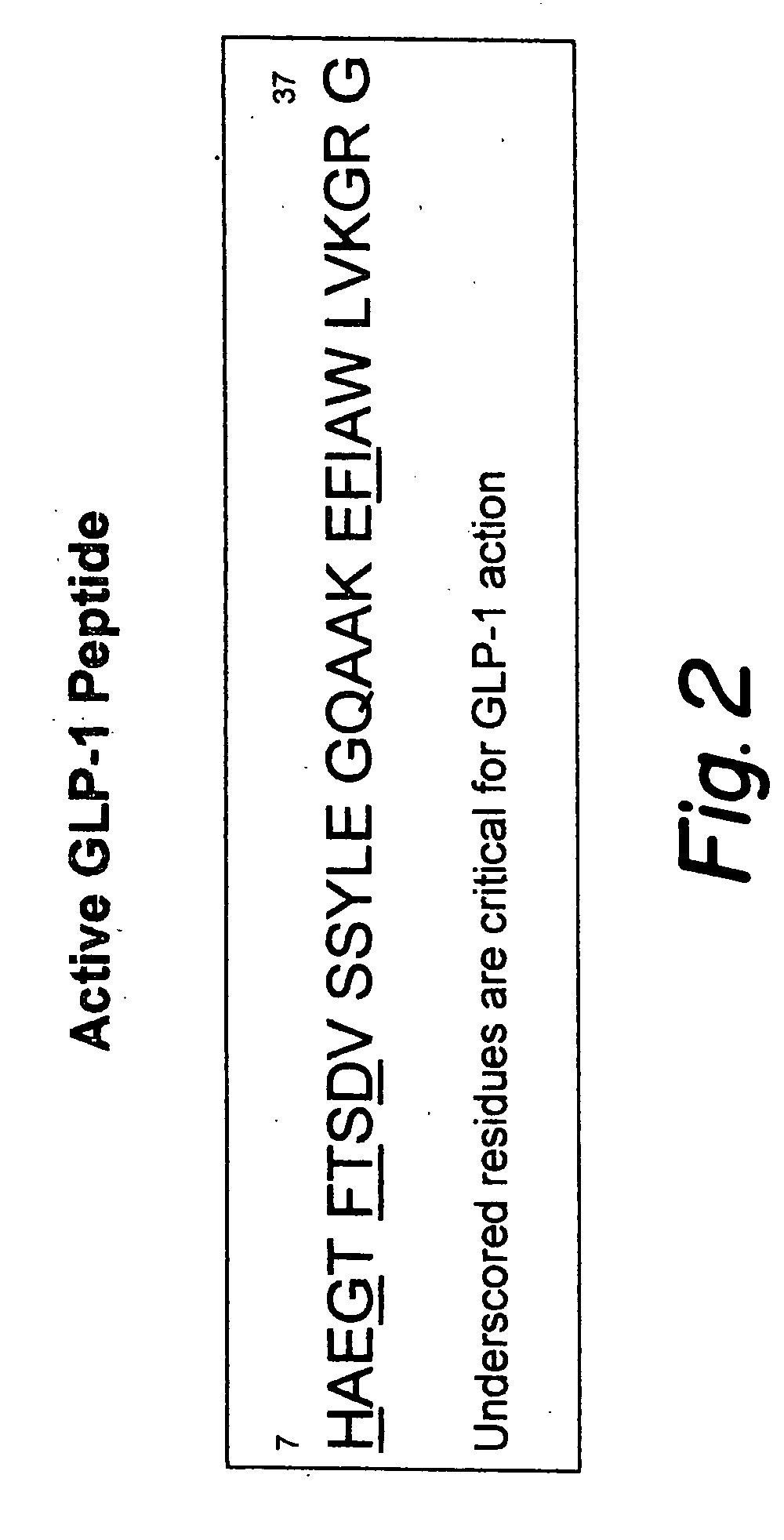
[0116]In order to obtain a biologically active form of secreted GLP-1 from genetically engineered cells, the following strategy is used to generate the GLP-1 nucleic acid constructs. The active GLP-1(7-37) peptide is comprised of 31 residues, accordingly the nucleotide sequence corresponding to these residues is conveniently split in half to generate two separate fragments viz., N-terminal and C-terminal fragments as shown in FIG. 3-B. Complementary oligonucleotides corresponding to these amino acids are synthesized with the modifications listed below. Sequences of the oligonucleotides are listed in SEQ ID NO:1, SEQ ID NO:2, SEQ ID NO:3 and SEQ ID NO:4.
[0117]An XhoI restriction site is added at the split site, without altering the amino acid sequence. A furin cleavage site (RQK′R) and the Kozak sequence and the bases consistent with the overhang of NheI restriction site are added to the 5′ end of the N-terminal fragment as shown in FIGS. 3-B and C. Bases consistent w...
example 2
Assessment of GLP-1 Secreted in Transiently Transfected Cells
[0123]Various human cell lines (293T, HeLa, SHP77, U2OS) are transiently transfected with GLP-1 constructs to assess production of GLP-1 in the culture medium. The transfections are performed as follows.
[0124]Cells are plated at 2.5×105 cells / 60 mm dish in 4.0 ml culture medium. The cells are allowed to adhere overnight. The cells are 40-60% confluent after the overnight culture. The medium is removed from the cells so that there is 1.5 ml remaining in the plate.
[0125]In a sterile microtube 1 μg DNA is added into a total of 150 μl DNA-condensation buffer (EC buffer, Qiagen Effectene transfection kit). Enhancer solution (8 μl l) is added into the DNA / EC buffer, vortexed for 1 second and incubated for 2-5 minutes at room temperature. 25 μl Effectene is added into each tube and vortexed for 10 seconds. The tubes are allowed to sit at room temperature for 5-10 min. The transfection mixture is diluted with 1.0 ml media and adde...
example 3
Engineering of Human U2OS Cells to Produce GLP-1
[0128]Human osteosarcoma cell line U2OS, available from the ATCC, is stably transfected with GLP-1 cDNA using the following protocol. The vector is linearized with BglII. The cells are co-transfected with pCDNA-huFurin expressing human furin. pCDNA-huFurin is linearized using MfeI. U2OS cells are seeded in 10-cm plates the day before at 2.5×105 per plate. The cells are transfected using calcium phosphate (Promega).
[0129]Three days post-transfection, medium is supplemented with 1000 μg / ml Geneticin. Once colonies are evident, cells are expanded in 6-well plates and tested for GLP-1 expression by active GLP-1 ELISA kit (Linco Diagnostic Services, Inc.). Cell populations producing high levels of GLP-1 are further cloned by limiting dilution at 1 cell / well. The clones are tested for their ability to produce GLP-1 in the presence and absence of DPPIV inhibitor.
[0130]The production of active GLP-1 in U2OS cells transiently transfected with n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| of time | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| nucleic acid sequence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com