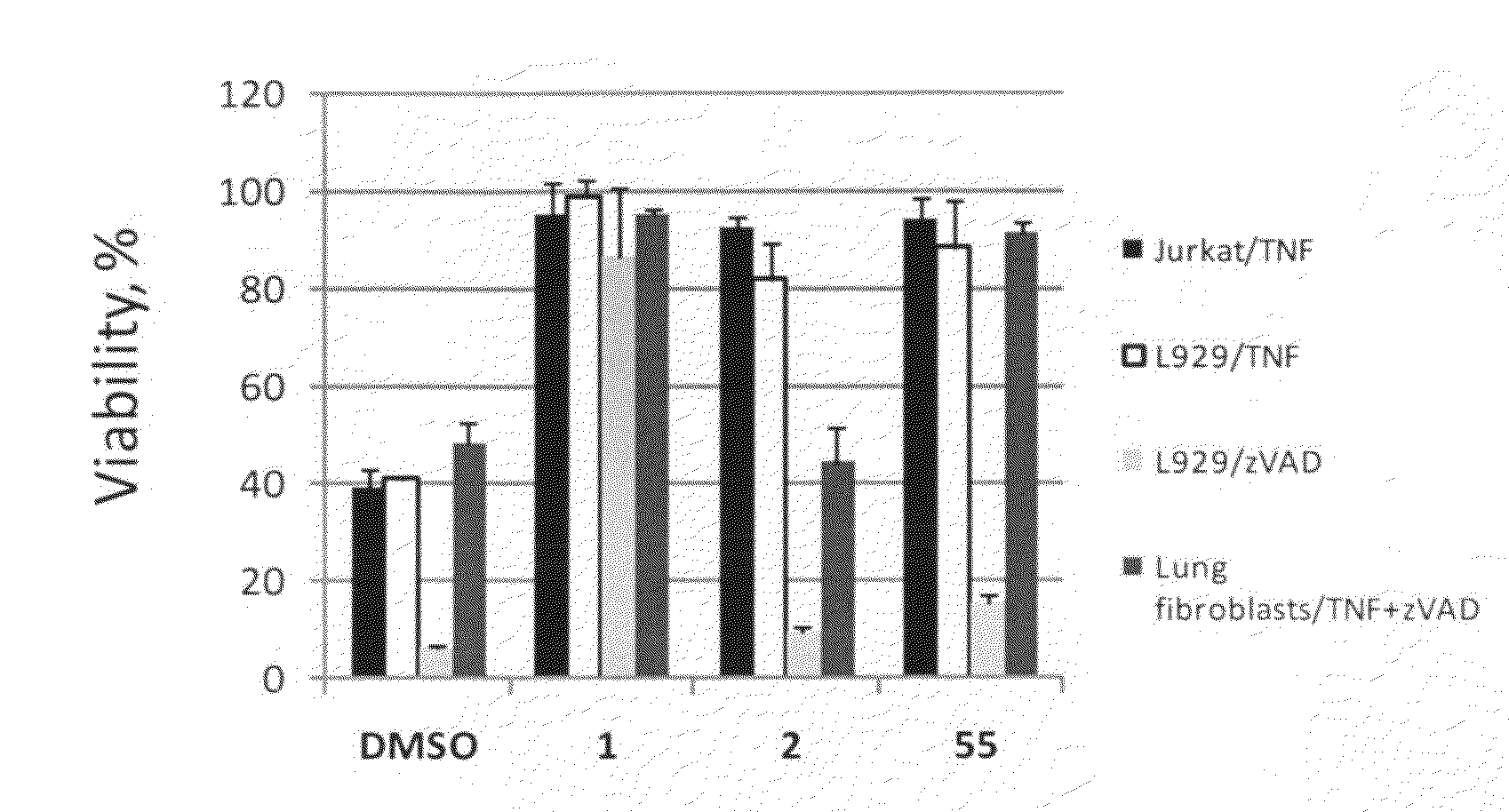
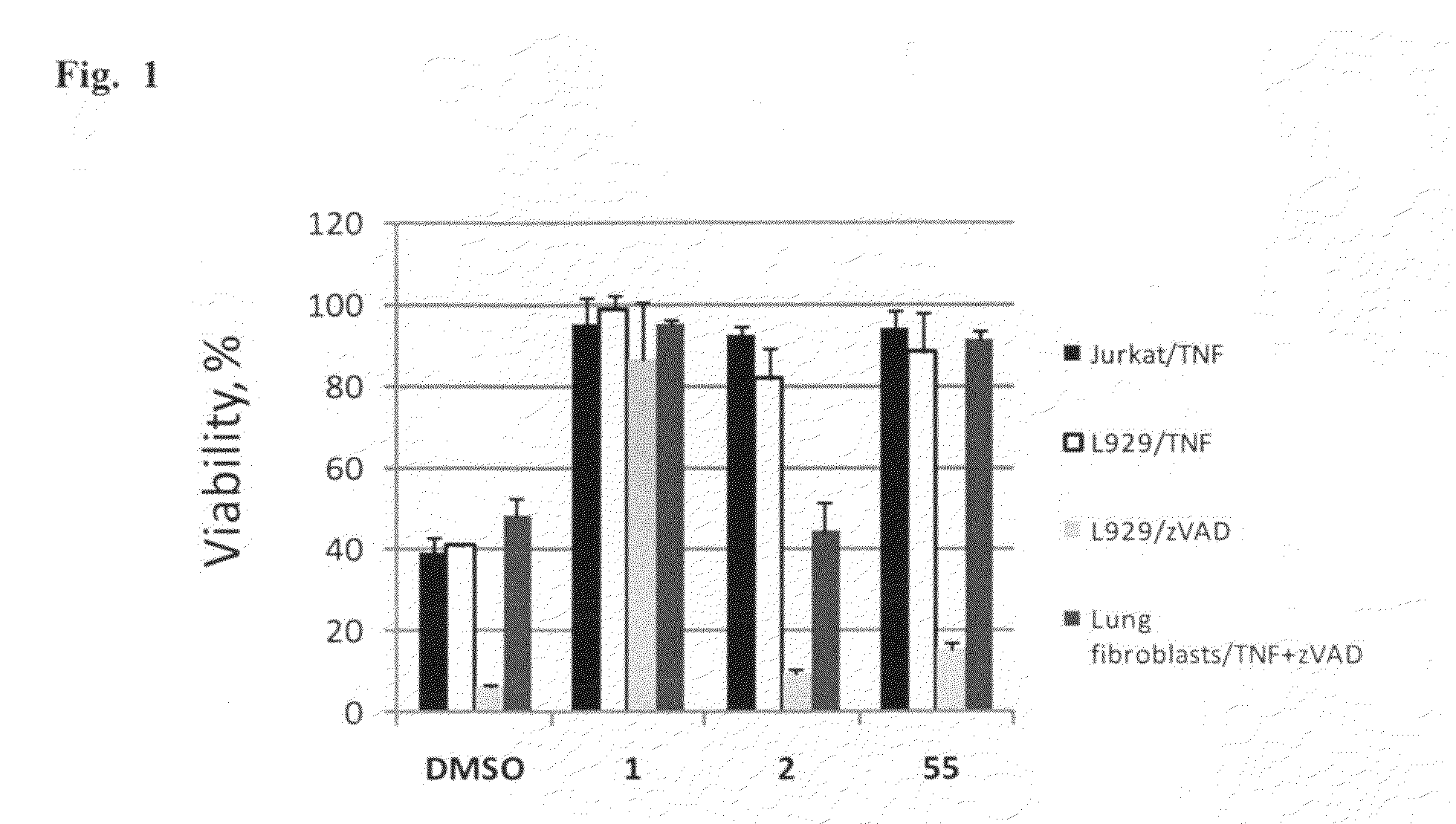
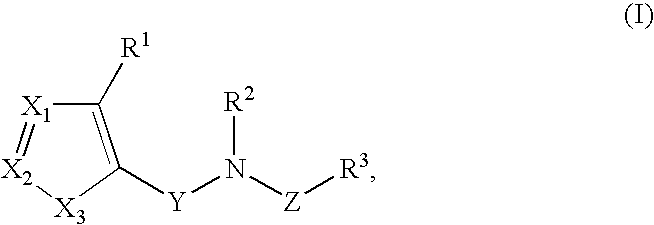
Heterocyclic inhibitors of necroptosis
a technology of heterocyclic compounds and necroptosis, which is applied in the field of heterocyclic compounds and to cell death, can solve the problems of complex underlying cell death mechanisms and insufficient control of necrosis, and achieve the effect of reducing necroptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of [1,2,3]thiadiazole derivatives of Formula (I-a)
[0177]The [1,2,3]thiadiazole derivatives are prepared according to the method outlined in Scheme 1. Meldrum's acid was treated with acyl chlorides in the presence of pyridine to give β-ketoester (step (a); Oikawa et al., J. Org. Chem. 43: 2087 (1978)). The esters were allowed to react with mono-Boc-hydrazine in the presence of a catalytic amount of p-toluenesulfonic acid (p-TsOH) to give the corresponding imines (step (b); Thomas et al., J. Med. Chem. 28: 442 (1985)). Cyclization in the presence of thionyl chloride yielded the [1,2,3]thiadiazole esters (step (c)). Base hydrolysis of the esters provided acids (step (d)). These materials were coupled with various amines utilizing HBTU (Method A), the corresponding acyl chlorides (Method B) or through the use of EDCI (Method C) to give amides of Formula (I-b).
example 2
Preparation of Compounds (13) and (16)
[0178]
[0179]Compound 13 was prepared according to the procedure outlined in Scheme 2. The ester was reduced with sodium borohydride (step (a)) and the product alcohol was converted to the corresponding aldehyde utilizing Dess-Martin reagent (step (b)). The aldehyde was condensed with 2-chloro-6-fluorobenzylamine in the presence of anhydrous magnesium sulfate to give an imine, which was subsequently reduced with sodium triacetoxyborohydride to give the secondary amine 13 (step (c)). The imide derivative 16 was also prepared starting with a carboxylic acid which was first converted to the corresponding acid chloride (step (d)). This material was then allowed to react with the anion of 2-chloro-6-fluorobenzamide generated with sodium hydride to give imide 16 in 34% yield (step (e)).
example 3
Preparation of α-substituted (±)-2-chloro-6-fluorobenzylamines
[0180]
[0181]The α-substituted (±)-2-chloro-6-fluorobenzylamines were prepared according to Scheme 3 (Polniaszek et al., J. Org. Chem., 55: 215 (1990)). 2-Chloro-6-fluorophenyl ketones were reduced with borane-tetrahydrofuran complex to give the secondary alcohols (step (a)). The alcohols were converted to the corresponding phthalimides via a Mitsonobu reaction (step (b)). The benzylamines were isolated following treatment with hydrazine monohydrate (step (c)). (S)-1-(2-Chloro-6-fluorophenyl)ethylamine was prepared by treating the benzonitrile starting material with methyl magnesium chloride followed by treatment with acetic anhydride to give α-enamide (step (d)). Asymmetric hydrogenation in the presence of the chiral catalyst (S,S)-Me-BPE-Rh gave the corresponding amide (step (e); Burk et al., J. Am. Chem. Soc. 118: 5142 (1996)). Acid hydrolysis of the amide yielded the optically pure amine (step (f)), isolated as the hyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell proliferation rate | aaaaa | aaaaa |
| Stereoisomer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com