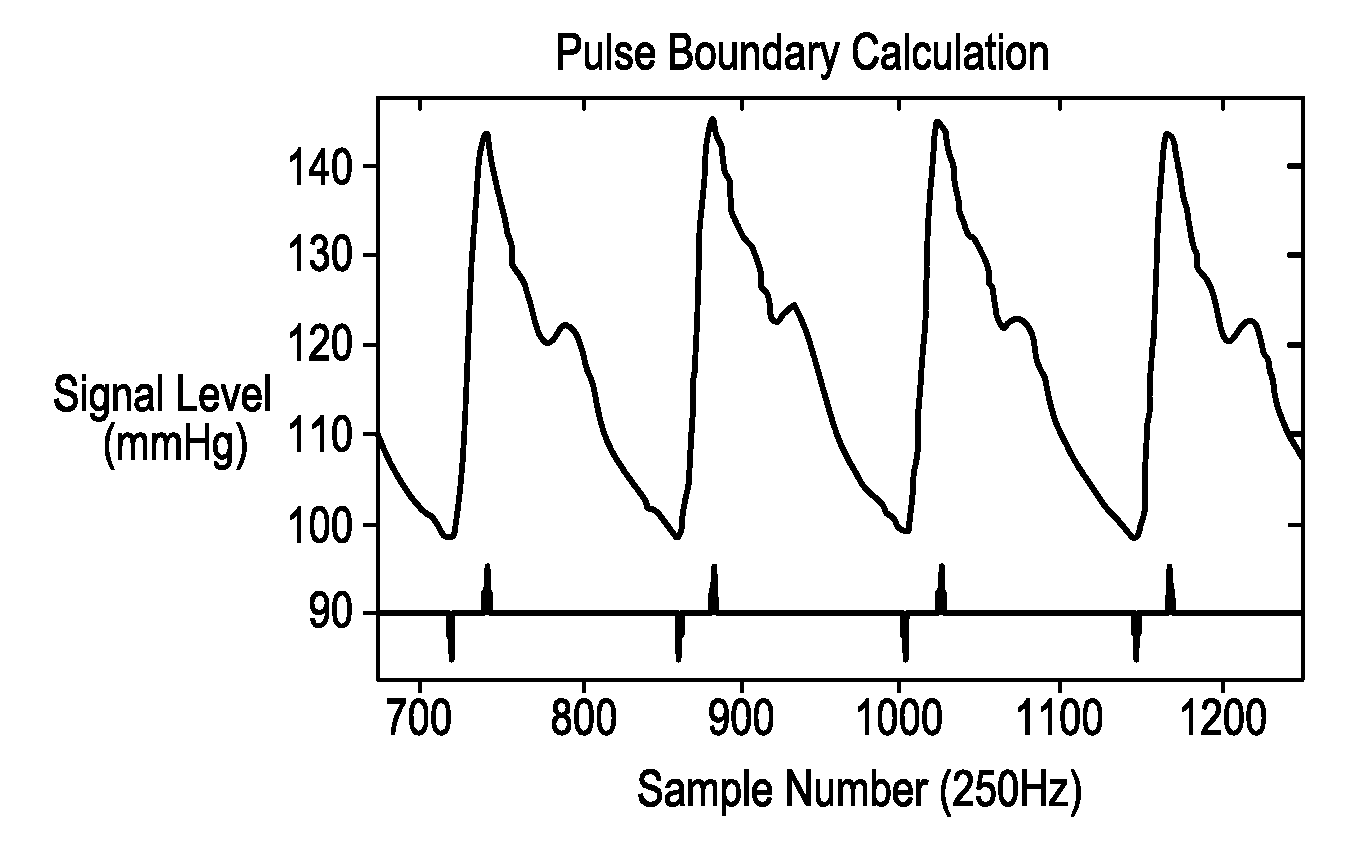
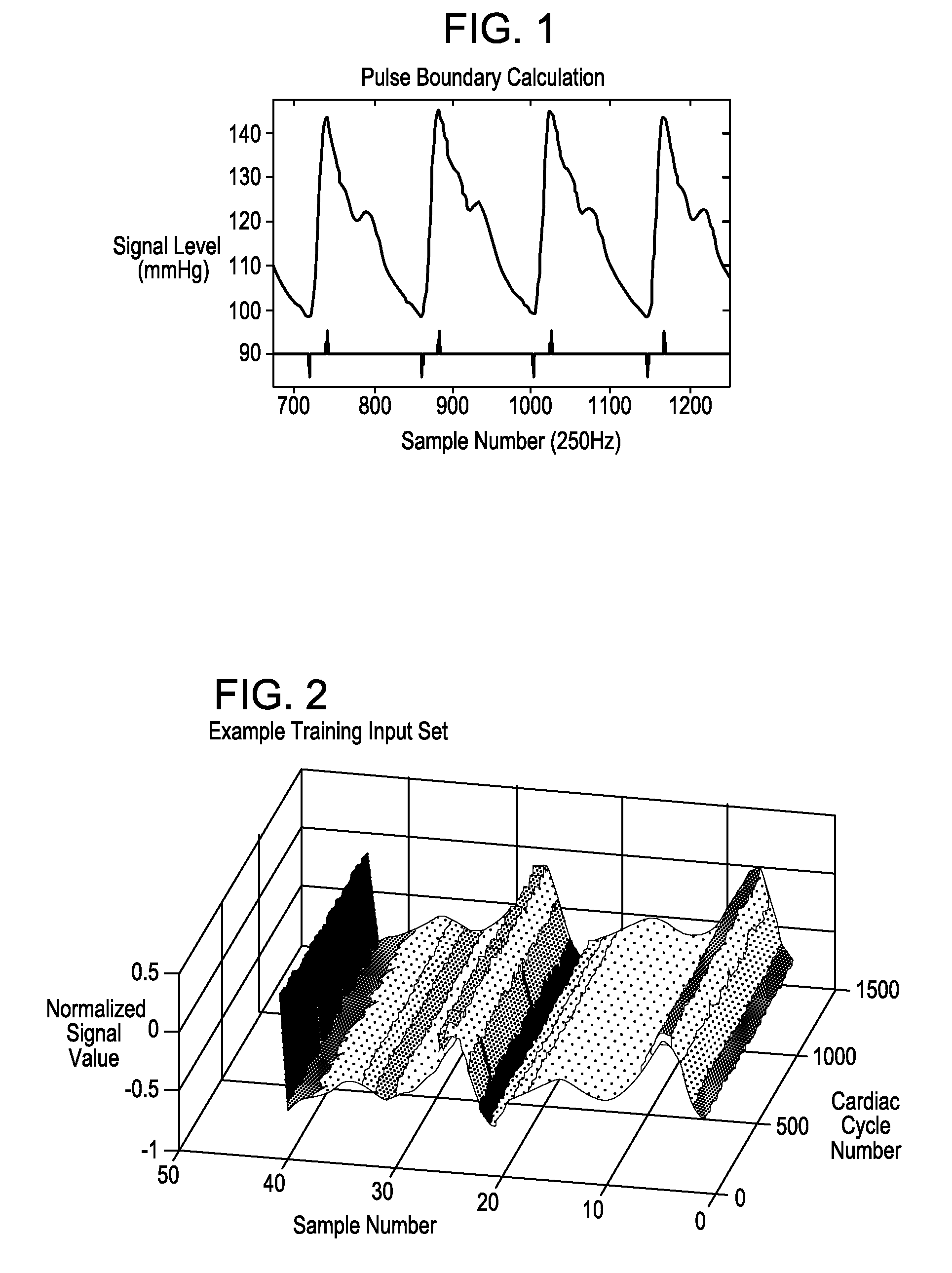
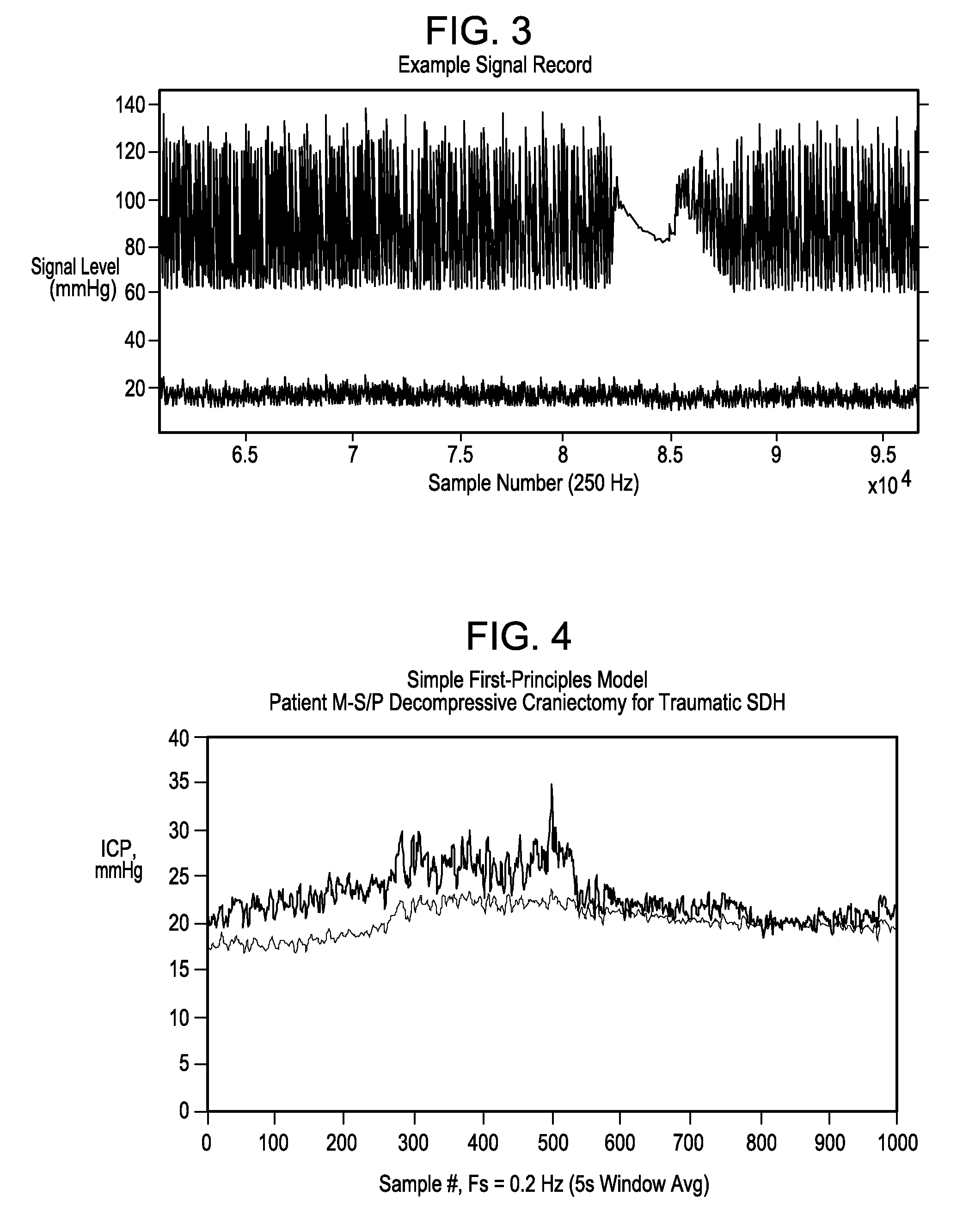
Systems and methods for determining intracranial pressure non-invasively and acoustic transducer assemblies for use in such systems
a technology of intracranial pressure and acoustic transducer, which is applied in the field of systems and methods for determining intracranial pressure non-invasively and acoustic transducer assemblies for use in such systems, can solve the problems of brain mechanical compression, herniation, and elevated intracranial pressure, and achieve accurate assessment and monitoring.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ICP Prediction Results Based on Empirical Studies Using TCD V_mca and Invasively Determined Continuous ABP Measurements as Variables
[0255]A prototype system for collecting data, deriving and applying a non-linear relationship between the variables of cranial blood vessel velocity and ABP was assembled using commercially available components. This prototype consisted of a notebook computer with a PCMCIA National Instruments (NI) 6024-E data acquisition (DAQ) card, a box containing the exposed backplane of the NI-DAQ card and a microphone input matching circuit, a specialized adapter designed to mate to the signal output port of a Spacelabs telemetry unit, and a Spencer Technologies TCD 100M Power M-Mode Digital Transcranial Doppler device and control pad with standard TCD ultrasound transducer and FDA-approved headband device for mechanical fixation to the head. The Spencer TCD 100M device was not modified in any way from its FDA-approved configuration. All electronic items were powe...
example 2
ICP Prediction Results Based on Empirical Studies and Training and Validation of an ANN
[0266]The prototype device described in Example 1 and the nICP determination methodology described in this specification was successfully tested on eighteen (18) patients at Harborview Medical Center in Seattle Wash., using blood pressure derived either directly from an arterial line or using arterial line-based ABP data simplified to mimic ABP data obtained from a blood-pressure cuff. A detailed description of the results for eight (8) of the eighteen patients was presented above in Example 1. Additional results are summarized below.
[0267]To determine the constants in the ICP prediction methodology, we acquired data from a set of patients (known as the ‘training set’) for whom we knew invasively measured ICP, as well as acoustic backscatter and ABP. For patients within the training set having a focal injury, their ICP was invasively measured from the same hemisphere as the injury focus, and the a...
example 3
[0275]Additional feasibility and efficacy testing of the methodology described above was performed using the experimental system described in Example 1. Acoustic backscatter, ABP and invasively measured ICP data was collected from a set of 25 patients (the ‘training set’). For training set patients having a focal injury, the ICP was invasively measured from the same hemisphere as the injury focus and acoustic backscatter was measured from this hemisphere as well. The acoustic backscatter data was collected from the MCA and MCA flow velocity values were derived from the acoustic backscatter using conventional Doppler techniques. An empirical algorithm was derived using this data and the neural network training protocol described above.
[0276]The derived algorithm was then tested in an iterative fashion to determine ICP for 21 patients using only acoustic backscatter and ABP data for the 21 validation patients for whom invasively measured ICP data had also been collected. The results o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com