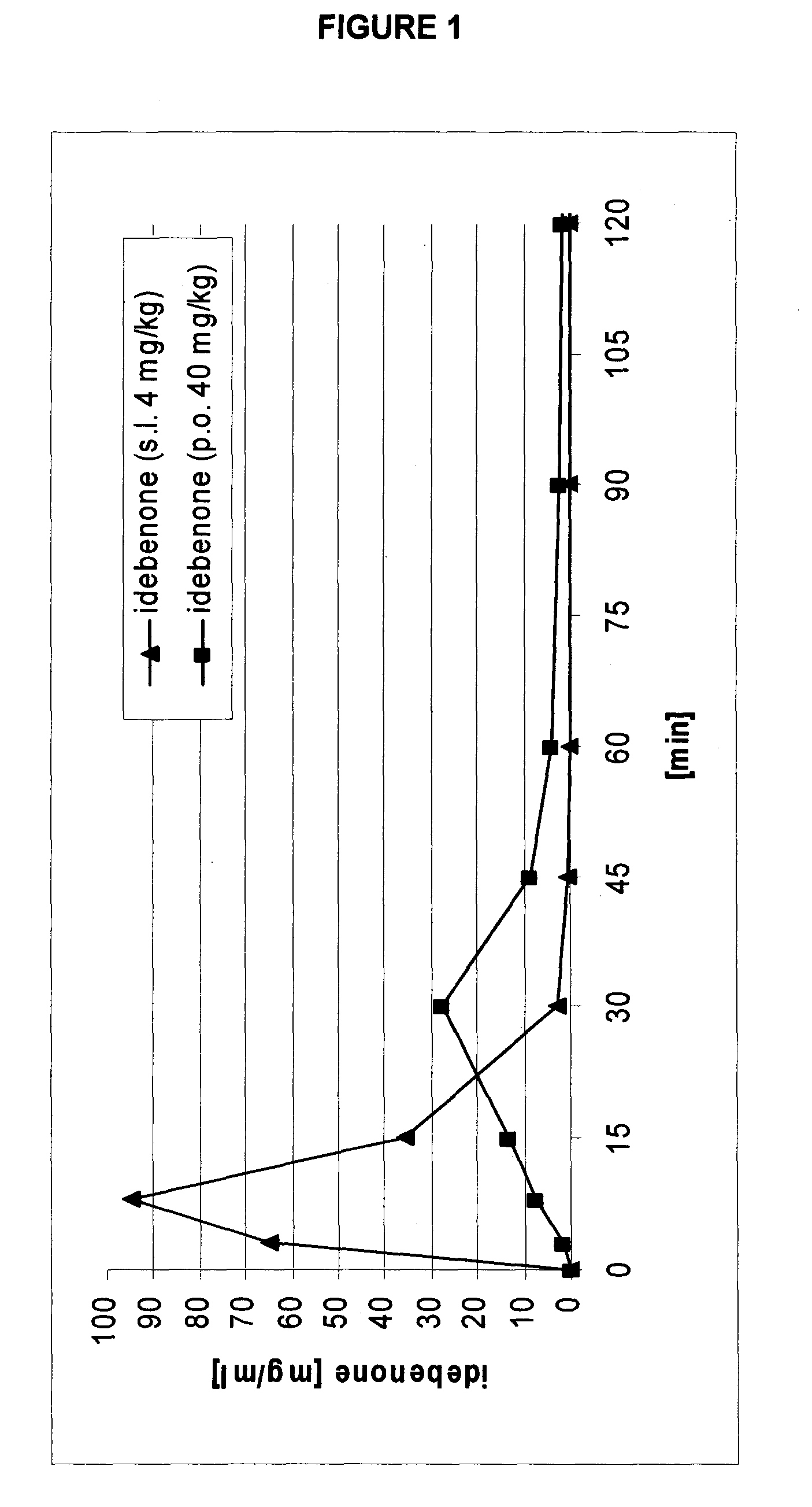
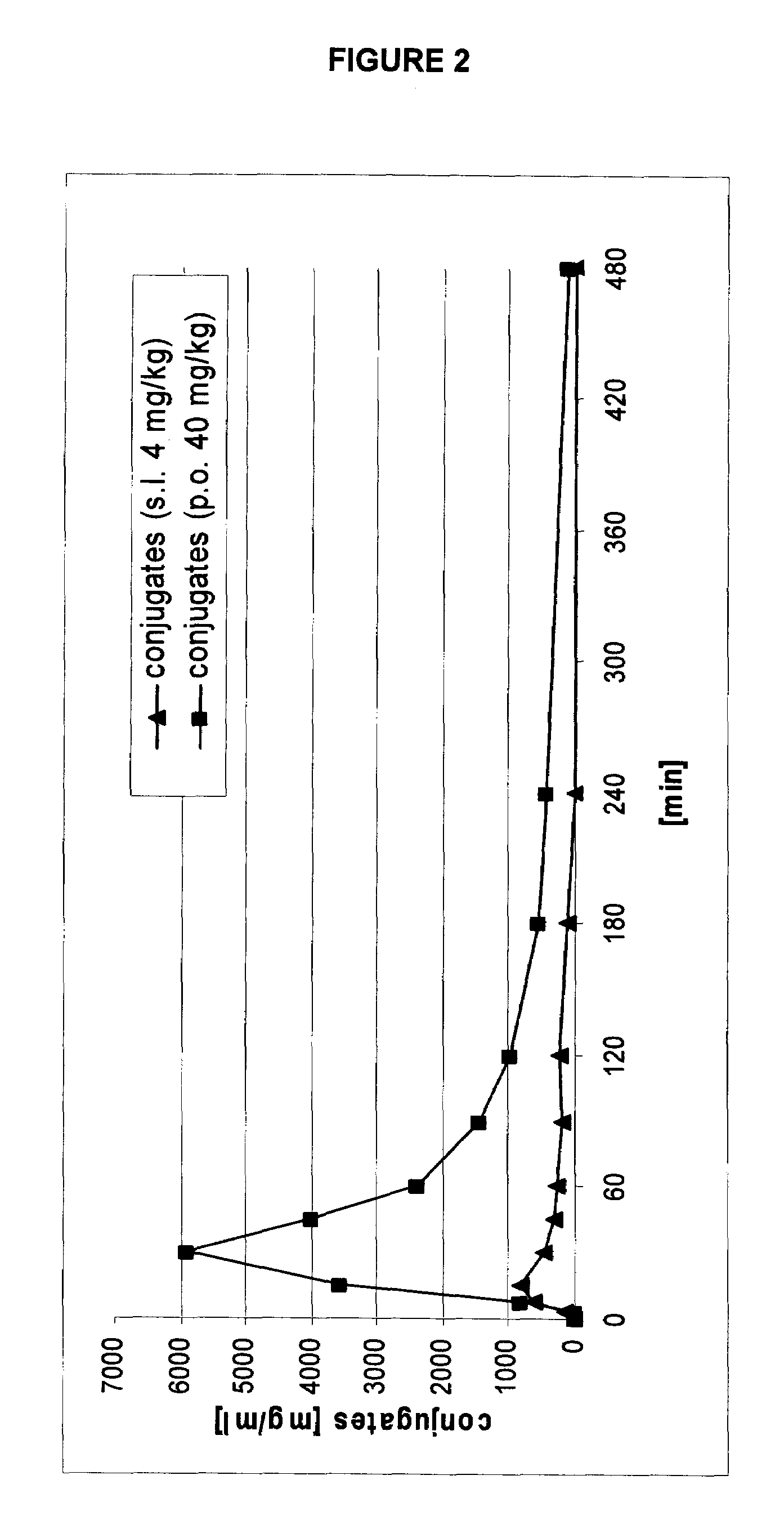
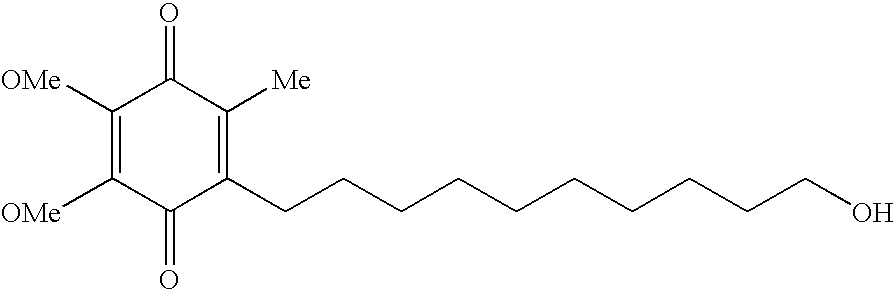
Transmucosal administration of 2,3-dimethoxy-5-methyl-6-(10-hydroxydecyl)-1,4-benzoquinone
a technology of idebenone and methyl-6, which is applied in the direction of antinoxious agents, drug compositions, metabolism disorders, etc., can solve the problems of poor the requirement for oral formulations of idebenone to be swallowed inflicts difficulties in the practical administration of patients with swallowing problems, and the dermal administration route has strong limitations regarding the administrable dose for systemic use, patient compliance and dosing accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Micro-Emulsion Formulation
[0031]
TABLE 1Ingredient[mg / ml]FunctionIdebenone20Active IngredientMiglyol 812 N144SolventTPGS96SurfactantWater750Solvent
[0032]Preparation: TPGS (Tocopherol-polyethylenglycol-400-succinate) is preheated to about 60° C. Miglyol 812 N and idebenone are mixed and sonicated for approx. 10 minutes until the substance is completely dissolved (clear orange solution). The molten TPGS is added to the Miglyol / idebenone solution and stirred. The mixture is diluted and homogenized until a homogeneous emulsion is obtained.
example 2
[0033]
TABLE 2Ingredient[mg / tablet]FunctionIdebenone10Active IngredientLactose monohydrate60FillerPovidone K304.5BinderCroscarmellose5DisintegrantMicrocrystalline cellulose30FillerFlavour2.5Flavouring agentAspartame6SweetenerMagnesium stearate0.75GlidantTalc1.25Glidant
[0034]Preparation: Idebenone, lactose monohydrate, flavor and aspartame are mixed in a high-shear mixer until a homogeneous mixture is obtained. Povidone is dissolved in water (approx. 8-10% solution) and added to the dry mixture and granulated. The wet granules are dried in a fluid bed dryer, sieved and added to a pre-mixture of microcrystalline cellulose, magnesium stearate and talc. The final mixture is compressed to tablets.
example 3
Spray Formulation
[0035]
TABLE 3IngredientamountFunctionIdebenone10gActiveMethylparahydroxybenzoate2gPreservativePropylparahydroxybenzoate0.2gPreservativeAspartame0.6gSweetenerFlavour0.6gFlavouring agentEthanol (96%)500mlSolventPurified waterq.s.Solvent
[0036]Preparation: 10 g Idebenone is dissolved in 500 ml ethanol (96%) and is mixed with 400 ml of purified water. Methylparahydroxy benzoate, propylparahydroxy benzoate and aspartame are dissolved in the mixture. The final volume is adjusted to 1000 ml with purified water. The solution is filtered and filled into an appropriate spray device.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| lipophilic | aaaaa | aaaaa |
| polar | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com