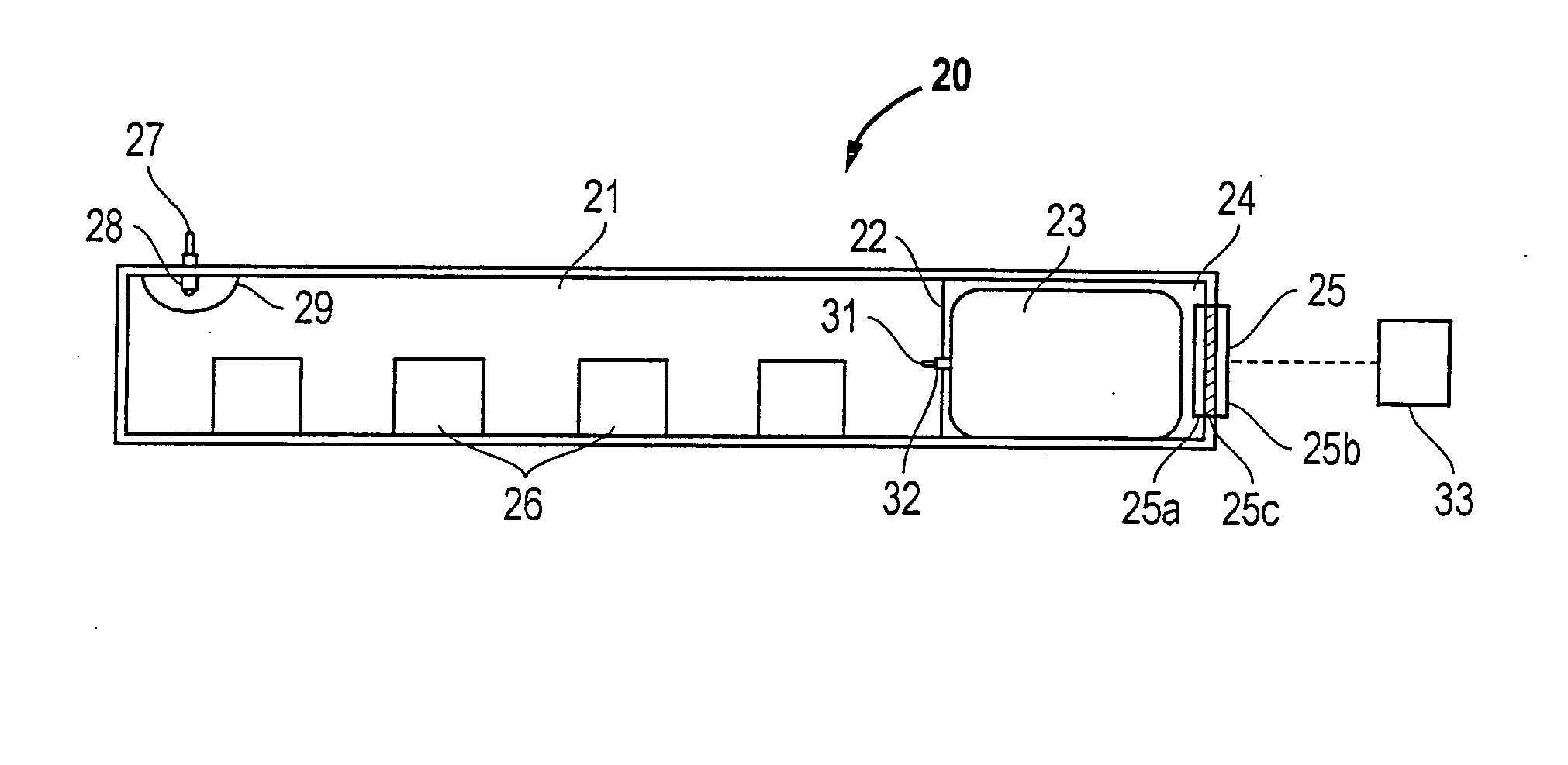
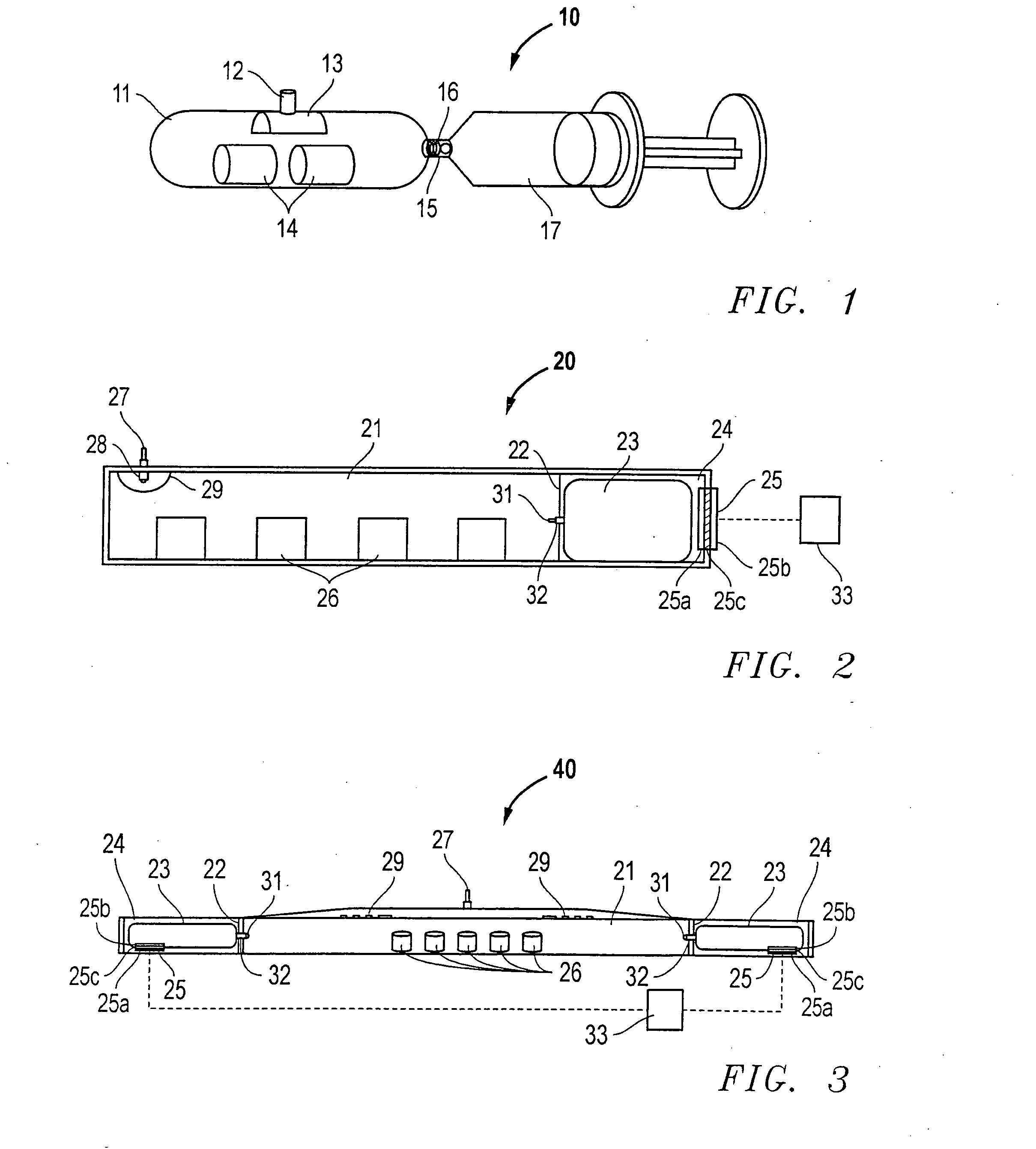
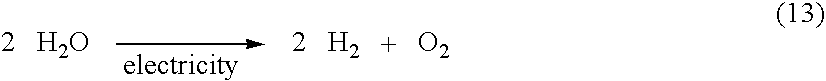Hydrogen generator
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Hydride Pellet Production
[0127]The hydride is frequently prepared as pellets. For each compound to be tested in this form, pellets were produced both neat and with predetermined amounts of catalyst blended with the hydride. For catalyzed pellets, the catalyst was blended with the hydride by grinding the components together. Pellets were standardized with a diameter of 13 mm and a height of ˜1 cm. The exact height of a pellet varied, as variations in additives and pressing conditions altered the final density. The pellets were produced using a standard pellet die (Graseby Specac) with a 12 ton press (Carver).
[0128]The effect on the density of lithium hydride pellets caused by varying the pressure exerted by the press is shown in Table 3. The accuracy of the pressures shown is about ±500 psi.
TABLE 3Pressure (psi)Density (g / mL)Fraction of Theoretical5,0000.53068.0%10,0000.55170.7%15,0000.57774.0%20,0000.60978.1%25,0000.64983.3%30,0000.65984.5%
[0129]All of the pellets showed good integr...
example 2
Evaluation of Hydrogen Evolution from Hydride Pellets
[0130]An apparatus for evaluating both neat and hydride-catalyst compositions for use in passively controlled generators is shown in FIG. 7. The hydride is shown as a pellet 26, which is a preferred form for the hydride because it is easily handled. A measured amount of water was injected into the flask 82 at the start of the experiment. Typically two to five grams of hydride were used in each reaction. The amount of water added was determined by the amount of hydride, the amount of water required to stoichiometrically hydrolyze it, and the stoichiometry being tested. As hydrogen was generated, the gas stream exited the flask 82, passed through a drying tube 85, and exited through a mass flow monitor 83 and vent 84. The drying tube 85 removed most, if not all, of the water in the gas stream. It is important that the dew point of the gas passing through the mass flow 83 is significantly below ambient to avoid condensate in the inst...
example 3
Hydride Pellets with Wicking Agents
[0133]LiH pellets were separately formed with four different wicking agents that included two sources of cellulose fibers, (paper and cotton), modified polyester having a surface treatment to enhance water transport along the surface without absorption into the fiber, and polyacrylamide, the active component of disposable diapers. In each case, the wicking material was included with the LiH in the die for pressing.
[0134]The pellets were hydrolyzed as described in Example 2. The fiber-containing pellets hydrolyzed quantitatively, unlike the results of Example 2. However, the reaction was quite rapid, lasting no more than a few minutes in any of the cases. The rate of hydrogen generation peaked in excess of 1.5 L per minute and then decreased to about 100 mL per minute within 5 minutes. The entire reaction was over in about 45 minutes.
[0135]In the presence of a ground paper wick or a polyacrylamide wick mixed into the hydrogen-containing composition ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
| Flow rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com