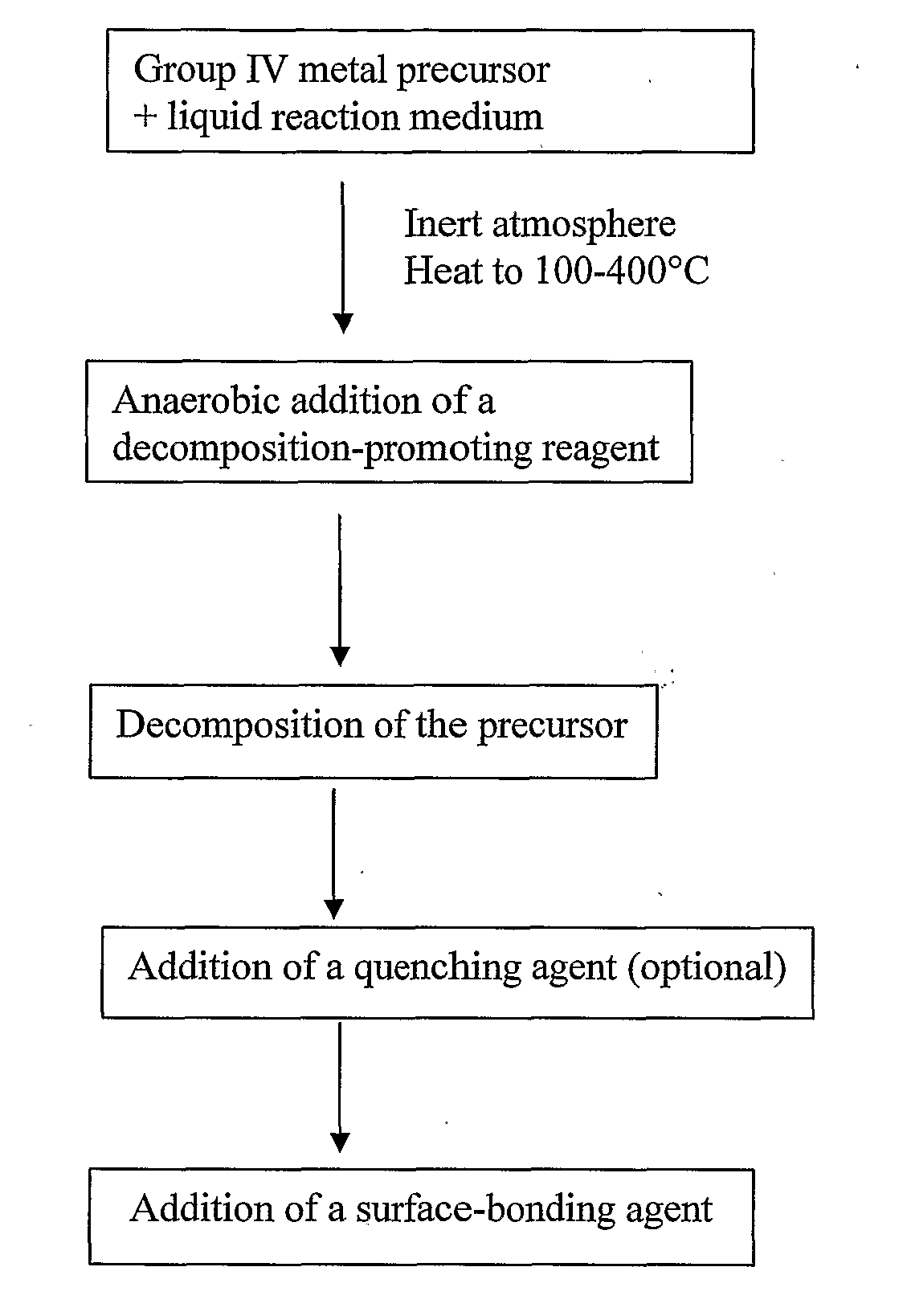
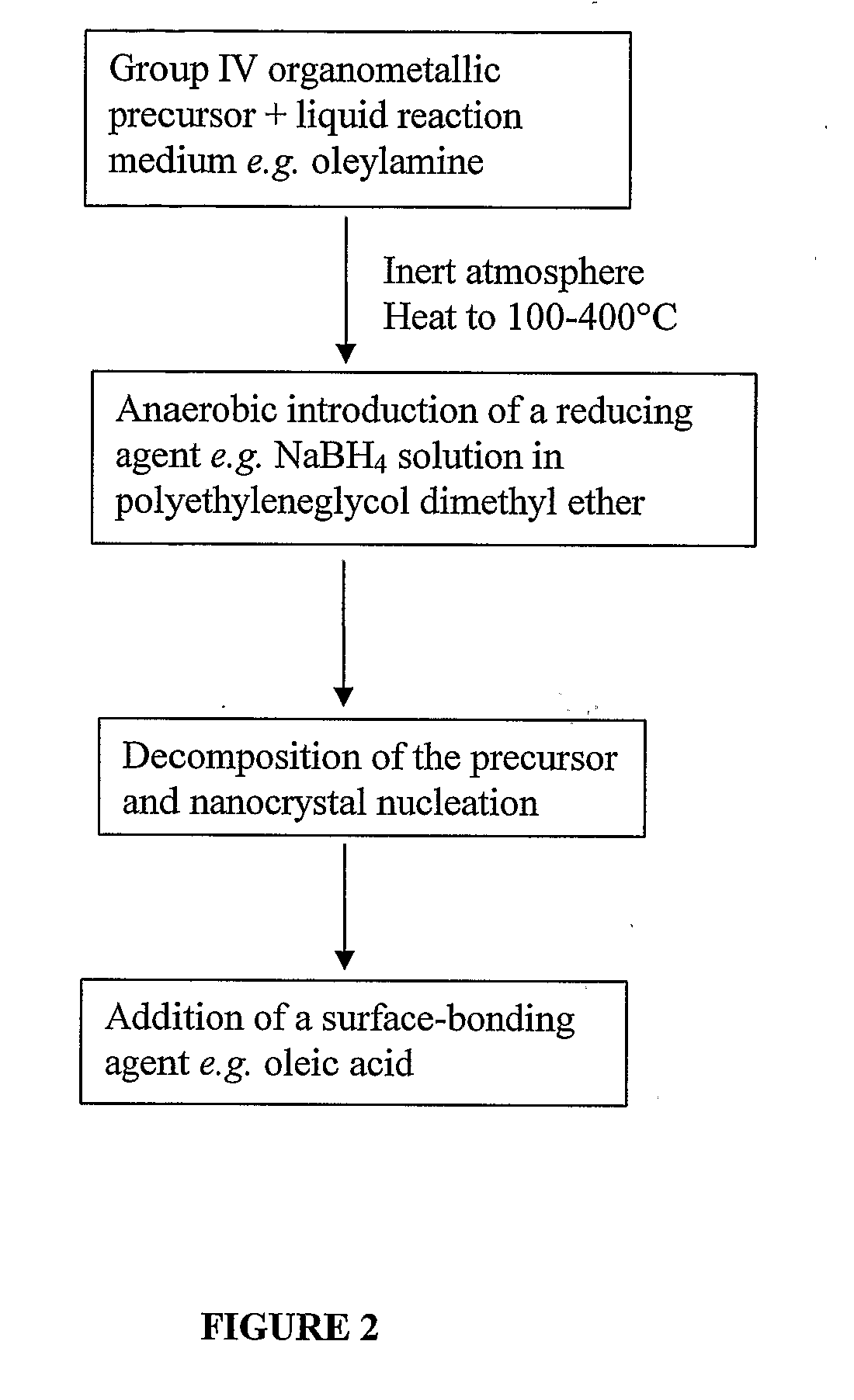
Methods of Forming Nanoparticles
a nanoparticle and nanoparticle technology, applied in the field of nanoparticle preparation methods, can solve the problems of high capital expenditure, low yield, and high cost, and achieve the effect of high luminescence and good monodispersity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0173]1) Place 4.5 g of hexadecylamine (HAD) or 7.5 ml of oleylamine in a three necked round bottom flask[0174]2) Add 0.05 g triphenylchlorogermane to flask[0175]3) Place flask in a stirring heating mantle under a cold water condenser and nitrogen purge flow[0176]4) Heat to 285-300° C. whilst stirring[0177]5) Inject a solution of 0.005 g sulfur in 1.5 ml trioctylamine[0178]6) Wait approximately 5 minutes[0179]7) Observe swift ([0180]8) Immediately add 0.2-5 ml oleic acid. The solution will rapidly clear[0181]9) Heat to a defined temperature between 285° C. and 360° C. for up to one hour[0182]10) Cool to 150° C. and then quench with a 1:1 mixture of ethanol and toluene, added dropwise[0183]11) Remove from flask. Add ethanol (for hexadecylamine) or methanol (for oleylamine) drop-wise until flocculation is observed[0184]12) Centrifuge out flocculated sediment and remove and keep supernatant[0185]13) Further dilute precipitate with flocculent and centrifuge. Repeat steps 9-11 until requ...
example 2
[0187]An alternative procedure omits steps (2) and (3) from the procedure described in Example 1 and instead utilises solutions of sulfur in oleylamine and triphenylchlorogermane in oleylamine which are injected into the heated solvent at a temperature between 260° C. and 360° C. The procedure is then followed as from step (5) of Example 1.
example 3
[0188]1) Place 4.5 g of hexadecylamine or 7.5 ml of oleylamine in a three-necked round bottom flask[0189]2) Add 0.012 g selenium to flask[0190]3) Place flask in stirring heating mantle under a cold water condenser and nitrogen purge flow[0191]4) Heat to 285-300° C. whilst stirring[0192]5) Inject solution of 0.05 g triphenylchlorogermane in 1.5 ml trioctylamine[0193]6) React for 30 minutes[0194]7) Cool to 150° C. and then quench with a 1:1 mixture of ethanol and toluene, added dropwise[0195]8) Remove from flask. Add ethanol (for hexadecylamine) or methanol (for oleylamine) drop-wise until flocculation is observed[0196]9) Centrifuge out flocculated sediment and remove and keep supernatant[0197]10) Further dilute precipitate with flocculent and centrifuge. Repeat steps 9-11 until required purity is reached. Steps 9-11 may also be repeated on the supernatant to yield size selective precipitation of nanoparticles[0198]11) Resuspend the final precipitated material in toluene, hexane or et...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com