System and method for ammonia synthesis
a technology of ammonia and synthesis method, which is applied in the field of ammonia synthesis, can solve the problems of high pressure and temperature, low reaction efficiency, increased operational and capital costs, etc., and achieves the effects of improving the function of nano-size catalyst particles, reducing the cost of operation and capital costs, and maintaining durability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
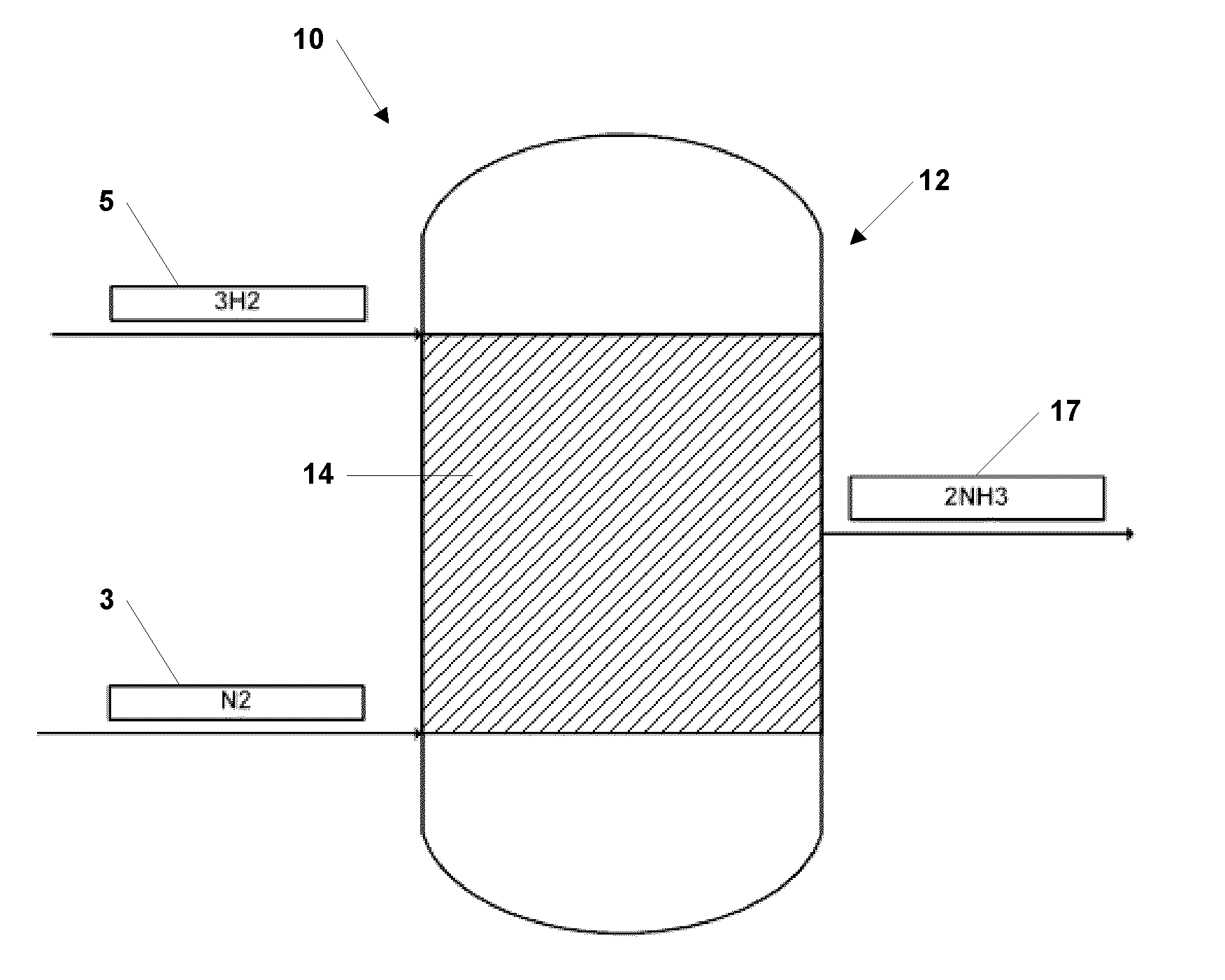
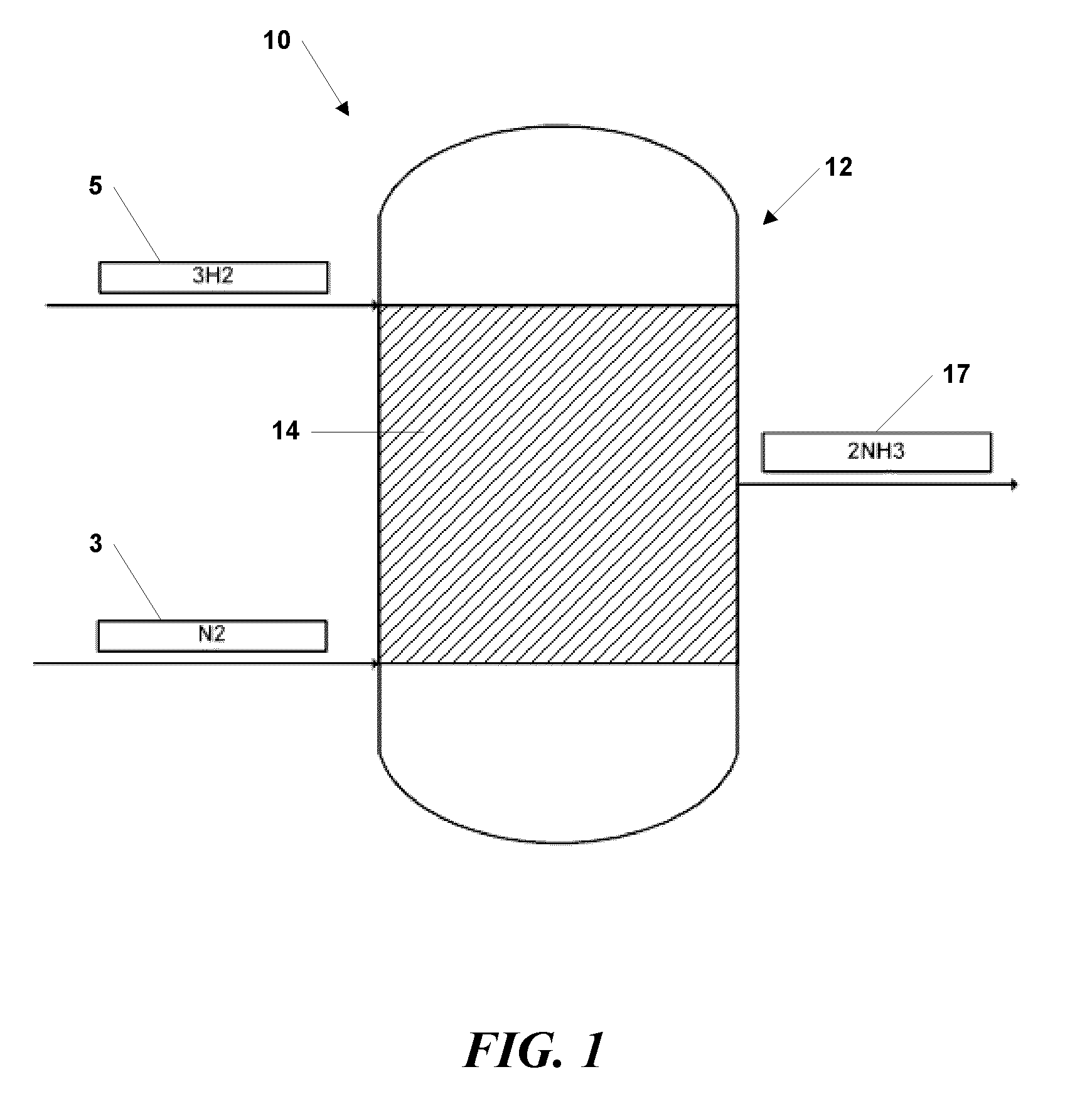
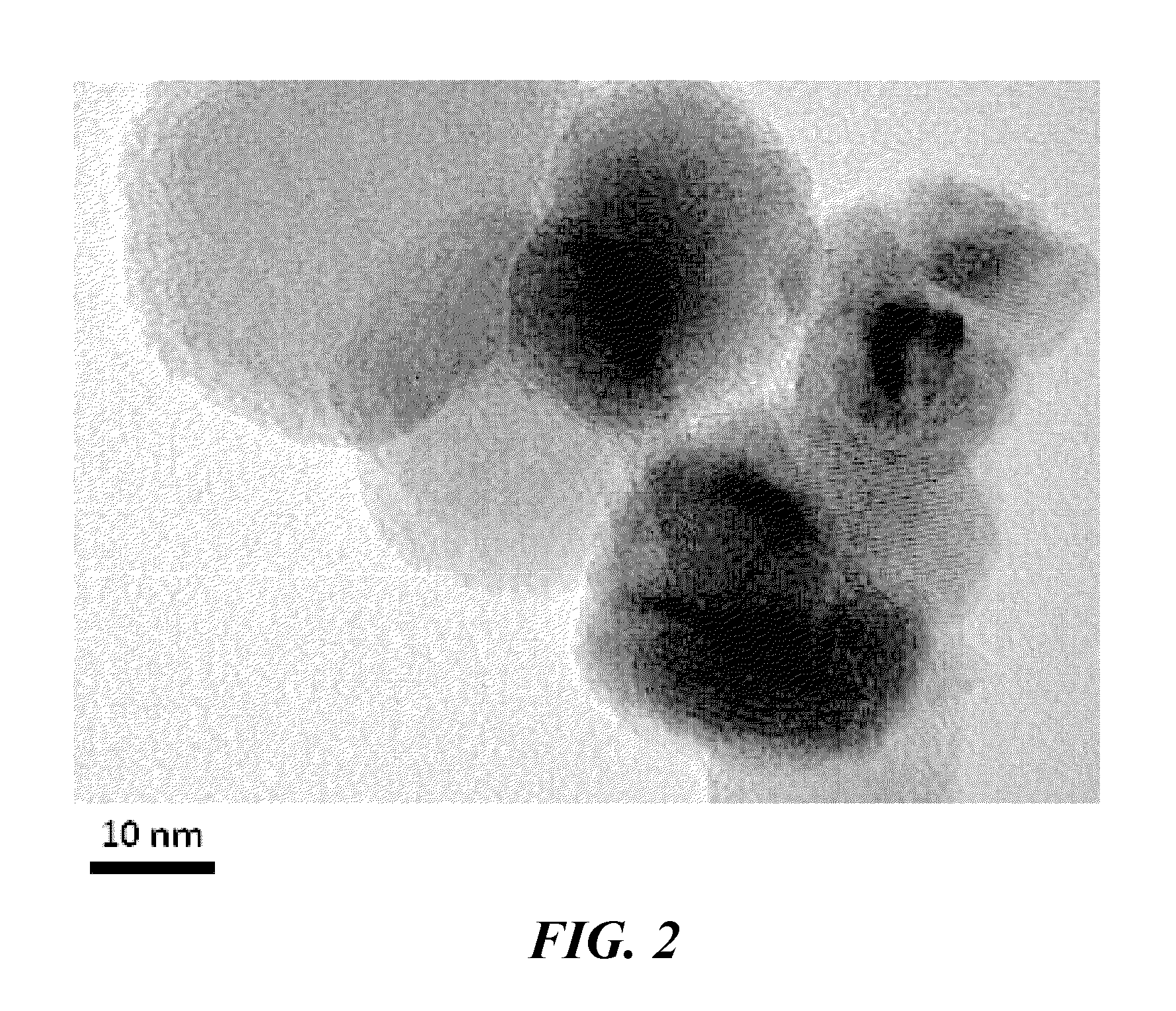
[0039]Synthesis of NH3 was performed over a bed of nano-sized ferrous catalyst particles, manufactured using the vapor condensation process described in U.S. Pat. No. 7,282,167 to Carpenter, and supported with silicon nitride tubes. The nano-sized ferrous catalyst particles comprised an oxide coating between about 0.5 and 1.5 nanometer thickness. The particles had average diameters from 15 to 25 nanometers.
[0040]The supported nano-sized ferrous catalyst particles were piled in a packed bed configuration within a plug flow reactor system. Hydrogen and nitrogen gases were introduced into to plug flow reactor system as described above at pressures between about 10 atm and 20 atm and a temperature of about 450° C.
[0041]Ammonia was detected and alkalinity tests conducted with pH paper yielded a pH of 11, typical of ammoniacal solutions in water. The experiment established the production of ammonia from hydrogen and nitrogen at vastly reduced pressures, as compared to industrial processes...
example 2
[0042]Synthesis of ammonia was performed over a bed of nano-sized ferrous catalyst particles, manufactured using the vapor condensation process described in U.S. Pat. No. 7,282,167 to Carpenter, and supported on SG9801R promoted iron from BASE. The nano-sized ferrous catalyst particles comprised an oxide coating between about 0.5 and 1.5 nanometer thickness rendering them air safe for mixing. The particles had an average diameter from 15 to 30 nanometers. The nano-sized iron and iron support particles were blended for 2 minutes at 20G with an acoustic mixer to distribute the nano-sized particles onto the support iron particles.
[0043]Supported nano-sized ferrous catalyst particles were piled in a packed bed configuration within a plug flow reactor system. The supported nano-sized iron particles were reduced in a stream of hydrogen gas at 300° C. Hydrogen and nitrogen gasses were introduced into the plug flow reactor system as described above at pressures between about 5 atm and 10 at...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| energy | aaaaa | aaaaa |
| adsorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com