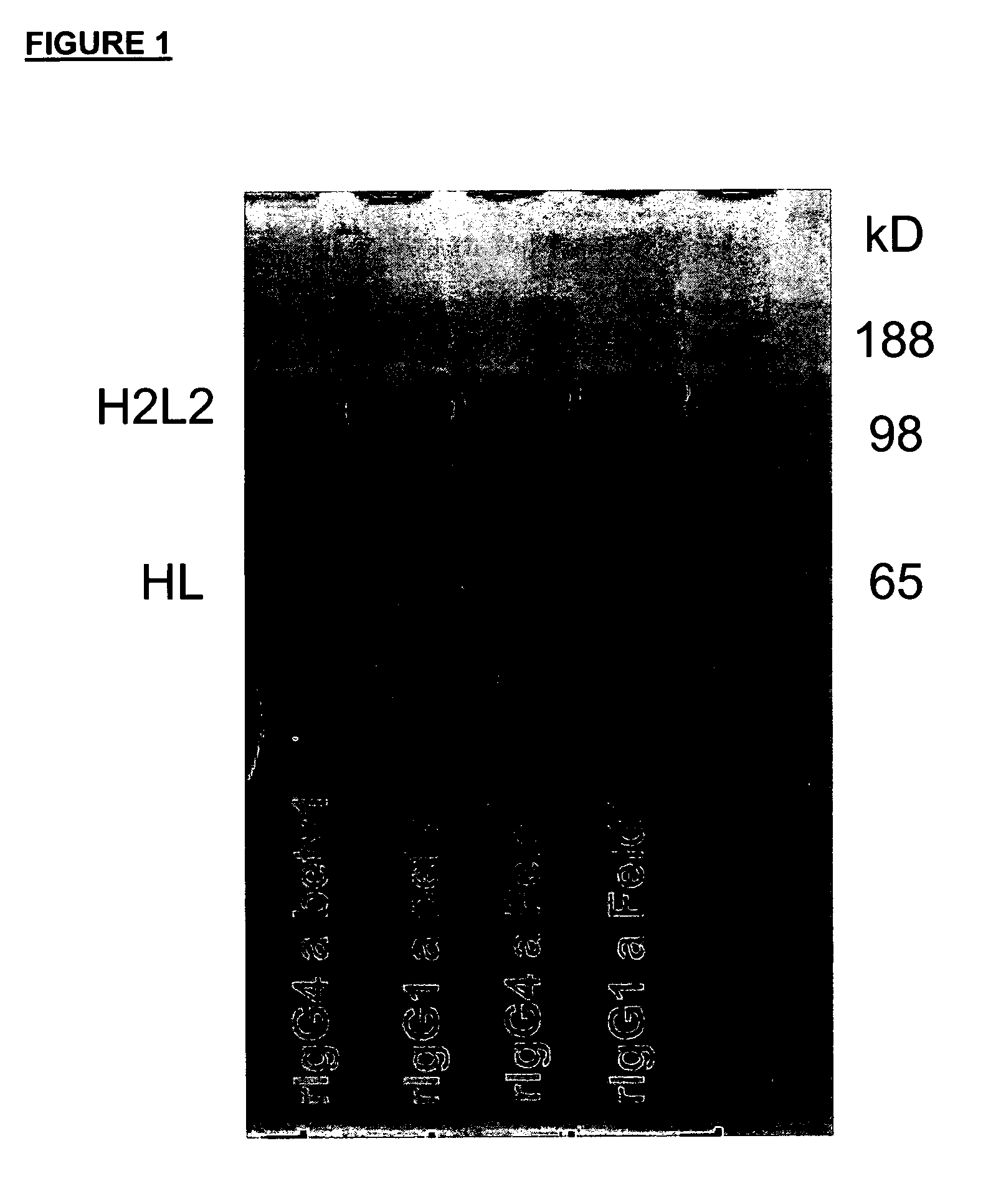
Stable igg4 antibodies
a technology of igg4 antibodies and stabilized antibodies, which is applied in the field of new stabilized igg4 antibodies to achieve the effect of reducing the risk of immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Oligonucleotide Primers and PCR Amplification
[0176]Oligonucleotide primers were synthesized and quantified by Isogen Bioscience (Maarssen, The Netherlands). Primers were dissolved in H2O to 100 pmol / μl and stored at −20° C. A summary of all PCR and sequencing primers is given below. For PCR, PfuTurbo® Hotstart DNA polymerase (Stratagene, Amsterdam, The Netherlands) was used according to the manufacturer's instructions. Each reaction mix contained 200 μM mixed dNTPs (Roche Diagnostics, Almere, The Netherlands), 6.7 pmol of both the forward and reverse primer, 100 ng of genomic DNA or 1 ng of plasmid DNA and 1 unit of PfuTurbo® Hotstart DNA polymerase in PCR reaction buffer (supplied with polymerase) in a total volume of 20 μl. PCR reactions were carried out with a TGradient Thermocycler 96 (Whatman Biometra, Goettingen, Germany) using a 32-cycle program: denaturing at 95° C. for 2 min; 30 cycles of 95° C. for 30 sec, a 60-70° C. gradient (or another specific annealing temperature) fo...
example 2
[0177]Agarose gel electrophoresis was performed according to Sambrook (Sambrook, Russell et al. 2000 Molecular cloning. A laboratory manual (third edition), Cold Spring Harbor Laboratory Press) using gels of 50 ml, in 1× Tris Acetate EDTA buffer. DNA was visualized by the inclusion of ethidium bromide in the gel and observation under UV light. Gel images were recorded by a CCD camera and an image analysis system (GeneGnome; Syngene, via Westburg B.V., Leusden, The Netherlands).
example 3
Analysis and Purification of PCR Products and Enzymatic Digestion
[0178]Purification of desired PCR fragments was carried out using a MinElute PCR Purification Kit (Qiagen, via Westburg, Leusden, The Netherlands; product #28006), according to the manufacturer's instructions. Isolated DNA was quantified by UV spectroscopy and the quality was assessed by agarose gel electrophoresis.
[0179]Alternatively, PCR or digestion products were separated by agarose gel electrophoresis (e.g. when multiple fragments were present) using a 1% Tris Acetate EDTA agarose gel. The desired fragment was excised from the gel and recovered using the QIAEX II Gel Extraction Kit (Qiagen; product #20051), according to the manufacturer's instructions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com