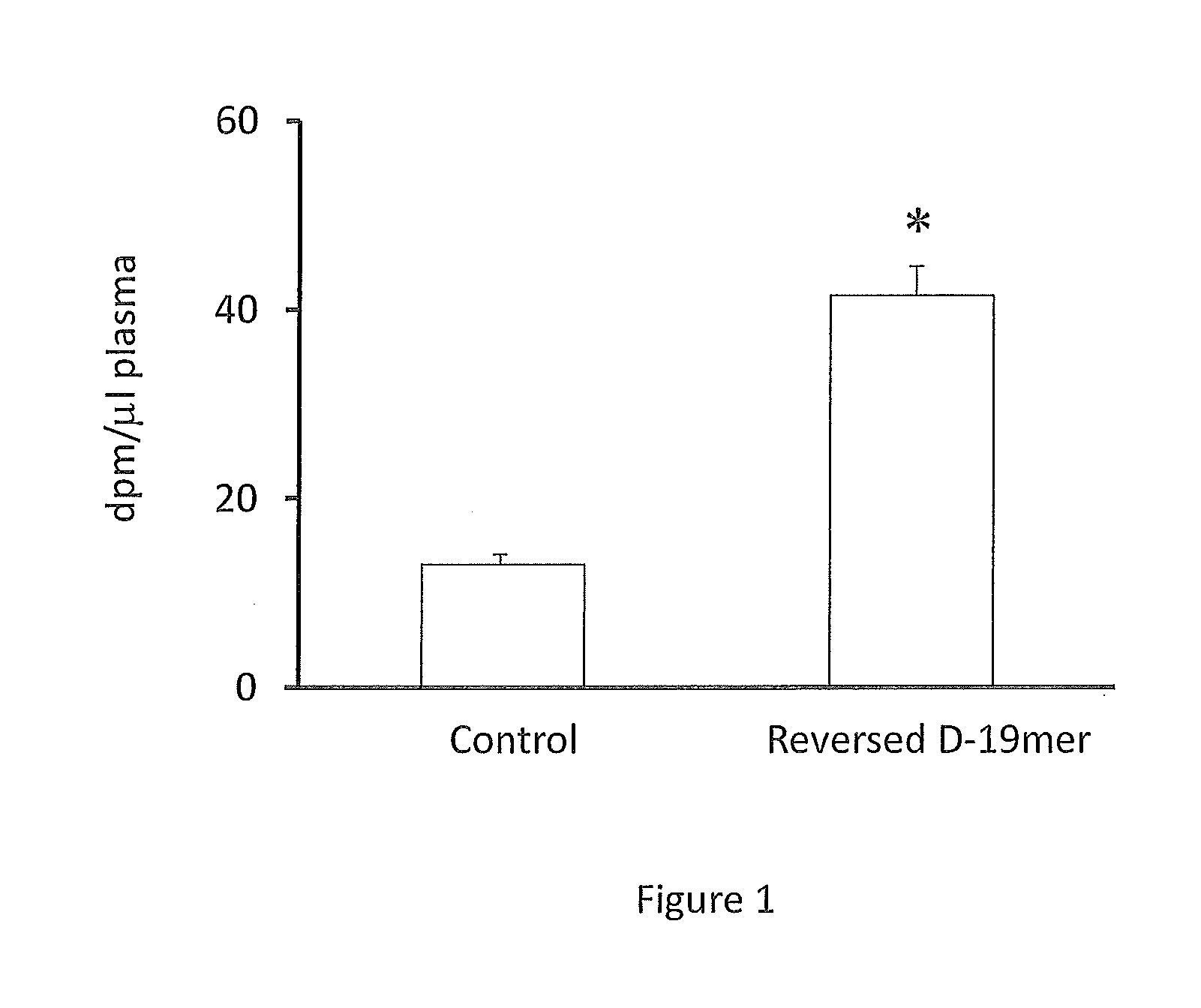
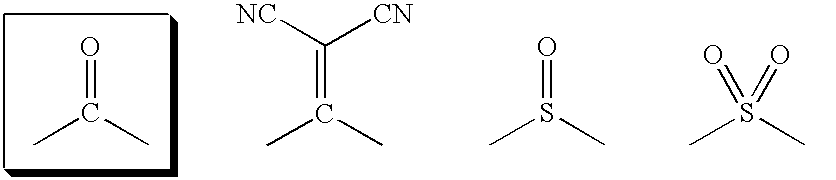
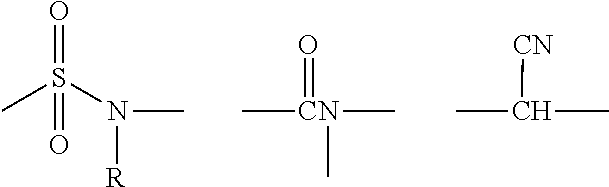
Compositions and Methods for Treating Atherosclerosis
a technology of reverse peptides and compound compounds, which is applied in the direction of peptide sources, animal/human peptides, metabolism disorders, etc., can solve the problems that reverse peptides do not always exhibit similar efficacy to their corresponding forward peptides, and achieve the effects of high density lipoprotein, potent enhancing effect on macrophage cholesterol ester hydrolase activity, and high permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Animals
[0074]Swiss-white CDl 6-8 week old female mice were obtained from Charles River, Montreal, Quebec. Mice were kept in a temperature controlled room on a 12 hour light / dark cycle. They were fed with Purina Lab Chow pellets and water ad libitum.
example 2
Chemicals
[0075]All chemicals were reagent grade and purchased from Fisher Scientific (Nepean, Ont.), Sigma (St. Louis, Mo.), ICN (Aurora, Ohio), or BioRad (Hercules, Calif.). Dulbecco's Modified Eagle's Medium (DMEM) and fetal bovine serum (FBS) were purchased from Life Technologies (Burlington, Ont.). Radiolabeled [1,2,6,7-3H(N)]-cholesterol (82 Ci / mmol) was obtained from DuPont NEN (Boston, Mass.).
example 3
Reverse Peptides
[0076]Peptides were synthesized by solid-phase chemistry using a PE Applied Biosystems 433A peptide synthesizer. The purity of the synthetic peptides was established by analytical high-performance liquid chromatography (HPLC) and ion spray mass spectrometry. The peptides were dialyzed against distilled water and lyophilized before use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com