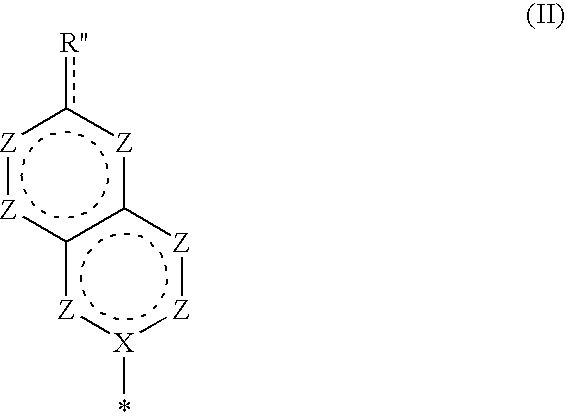
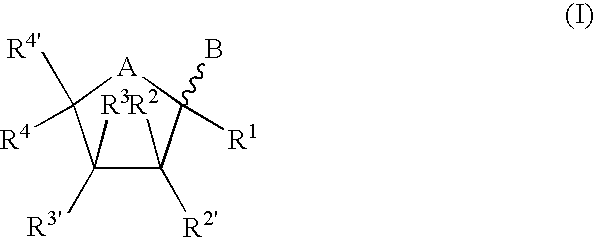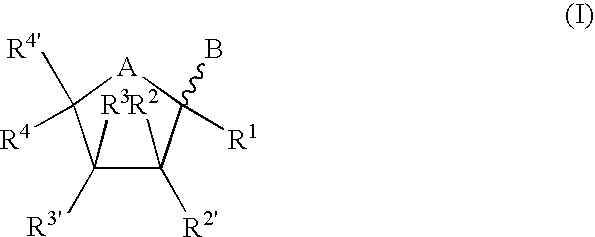Bicyclic nucleosides and nucleotides as therapeutic agents
a technology of bicyclic nucleosides and nucleotides, which is applied in the direction of biocide, plant growth regulators, sugar derivatives, etc., can solve the problems of mutagenic damage to the viral genome, limited disclosure of bicyclic nucleosides, and major threat to human health from viral infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Synthesis
[0232]
TABLE 1ExampleStructureMolec FormulaMWt1C13H15N3O6309.282C13H14IN3O6435.173C13H14BrN3O6388.174C13H14ClN3O6343.725C13H15N3O6309.286C14H17N3O6323.307C20H21N3O6399.408C16H21N3O6351.369C14H19N3O6325.3210C20H23N3O6401.4211C17H24N4O5364.4012C17H24N4O6380.4013C18H26N4O5378.4314C17H25N5O5379.4115C13H18N3O15P3549.21
Experimental Data
[0233]1H and 31P NMR spectra were recorded on either a Bruker Avance DRX 400, AC 200 or AM 300 spectrometer. Spectra were recorded in CDCl3, d6-acetone, CD3OD or d6-DMSO using the residual solvent peak as a reference. Chemical shifts are reported on the δ scale in parts per million (ppm) using the following conventions to assign the multiplicity: s (singlet), d (doublet), t (triplet), q (quartet) m (multiplet) and prefixed b (broad). Mass spectra (ESI) were recorded on a Finnigan LCQ Advantage spectrometer. All microwave reactions were carried out in a CEM Discover microwave reactor. Flash and radial chromatography was performed on 40-63 μm silica...
preparation of examples 1-4
[0241] Preparation of Example 1 is an example of the general method.
example 1
[0242] Intermediate B (60 mg) was suspended in dry methanol (1 mL) under argon. A freshly prepared solution of 1M sodium methoxide in methanol (0.5 mL) was added and the reaction stirred for 18 hrs. Evaporation of the solvent in-vacuo with minimal heating and purification by radial chromatography on silica eluting with 20% MeOH / DCM yielded the required compound as a crystalline solid.
[0243] MS m / z ([M+H]+) 309.9. 1H 1H NMR (CD3OD+D2O) δ 9.24 (s, 1H), 7.61 (d, 1H), 6.23 (d, 1H), 6.11 (s, 1H), 4.08-4.00 (m, 2H), 3.93-3.83 (m, 2H), 1.12 (s, 3H).
[0244] Similarly for Examples 2 and 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com