Antibodies against influenza virus and methods of use thereof
a technology of antiviral antibodies and influenza viruses, applied in the field of antiviral antibodies, can solve problems such as the substantial risk of a new pandemic to aris
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
General Methods
Expression and Preparation of Various Panning Antigens for Selection of Phage Display Antibody Library.
[0180]HA1. HA1 is the N-terminal fragment (aa17-338) of (A / Thailand / 2(SP-33) / 2004(H5N1). The gene was codon-optimized and expressed as fusion protein with a C-terminal 9 amino-acids tag (C9-tag:GTETSQVAPA). The fusion protein HA1-C9 was expressed in 293T cells transiently and the secreted protein was purified from supernatant by affinity chromatography. Protein A Sepharose covalently coupled with anti-C9 antibody 1D4 (National Cell Culture Center) was used for purification of HA1-C9.
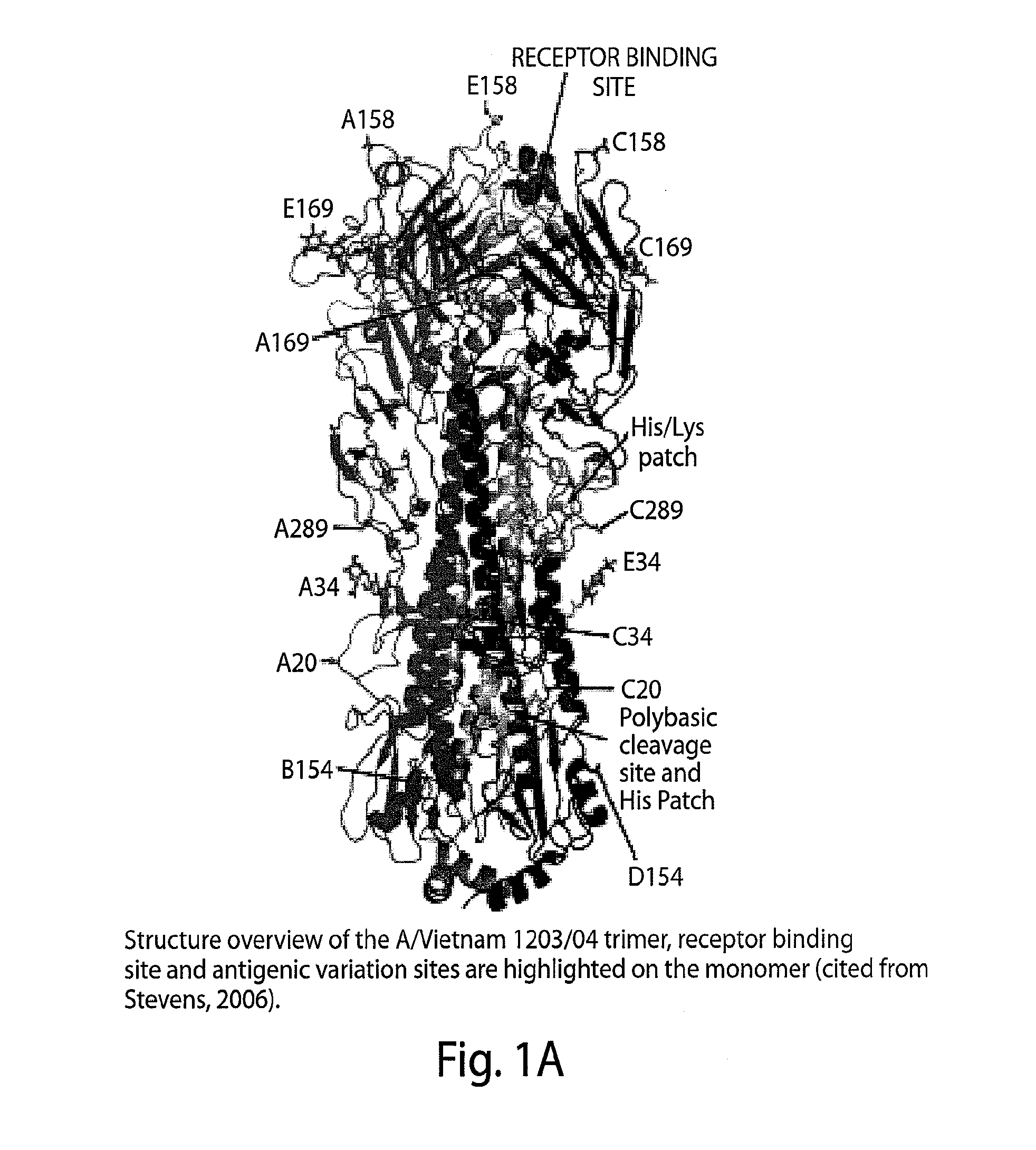
[0181]Trimeric HA0. The ectodomain (HA0) of hamagglutinin (HA) gene of A / Viet Nam / 1203 / 2004 was expressed in insect cells as a fusion protein by adapting the protocol described previously33. This construct contains a C-terminal trimerizing ‘foldon’ sequence from the bacteriophage T4 fibritin to stabilize the trimeric structure, followed by a thrombin site and a His6 tag. The cDNA of the f...
example 2
Identification of Neutralizing Antibodies Against H5N1 Using Viruses Pseudotyped with H5-SP33 and Microneutralization Assay
Results of Neutralization Assay Using Pseudotyped Viruses
[0211]Bivalent scFvFcs of anti-H5 antibodies were produced and tested for neutralization activities against HA0-pseudotyped viruses. We found that 2A is a moderate neutralizing antibody, 38B and 1C are non-neutralizing antibodies (data not shown). The 2A antibody has been used as a useful anti-HA reagent for other assays (epitope mapping) described below.) By using HA0 trimer as a target, we have identified 10 new unique antibodies against HA0. These Abs were screened for neutralization activity by using a novel high-throughput phage-scFvs screening method that has been recently set up in our lab. Briefly, the phage-scFvs of individual clones were directly used in a neutralization assay immediately following being screened by ELISA for positive clones in 96-well plates. In this schema, neutralization activ...
example 3
Kinetic Characterization of the Binding of HA0 (the Ectodomain of HA of A / Vietnam1203 / 04) to D8-IgG1, F10-IgG1 and A66-IgG1 by using BIAcore T-100
[0216]Mabs were captured on a CM4 chip via anti-human IgG1, and trimeric HA0 at various concentrations (20, 10, 5, 2.5, 2.5, 1.25, 0.625 nM) was injected over the chip surface. Binding kinetics was evaluated using a 1:1 Laugmuir binding model. The recorded binding curves (with blank reference subtracted) are shown in black and the calculated curves in red. All ka, kd, KD represent the means and standard errors of three experiments. Using Biacore T100 and Biacore T100 evaluation software, we determined the kinetic rates and affinity constants for the three anti-HA MAbs by fitting to a 1:1 Langmuir model. As shown in the figure below, similar ka, kd, and KD of D8 and F10 were observed, and A66 had a 1.8 fold lower KD than 8 and 10 due to a relative faster dissociation rate than the other two antibodies. The complexes between Mabs and HA0 wer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Exposure limit | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com