Antiviral composition comprising a sulfated polysaccharide
a technology of sulfated polysaccharides and compositions, applied in the field of sulfated polysaccharides, can solve the problems of inability to uniformly infect the immune system, the mechanism of sulphated polysaccharides, especially carrageenans, and the inability to inhibit viral replication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Effect of Different Concentrations of Iota-Carrageenan on Influenza A Virus Plaque Formation in MDCK Cells
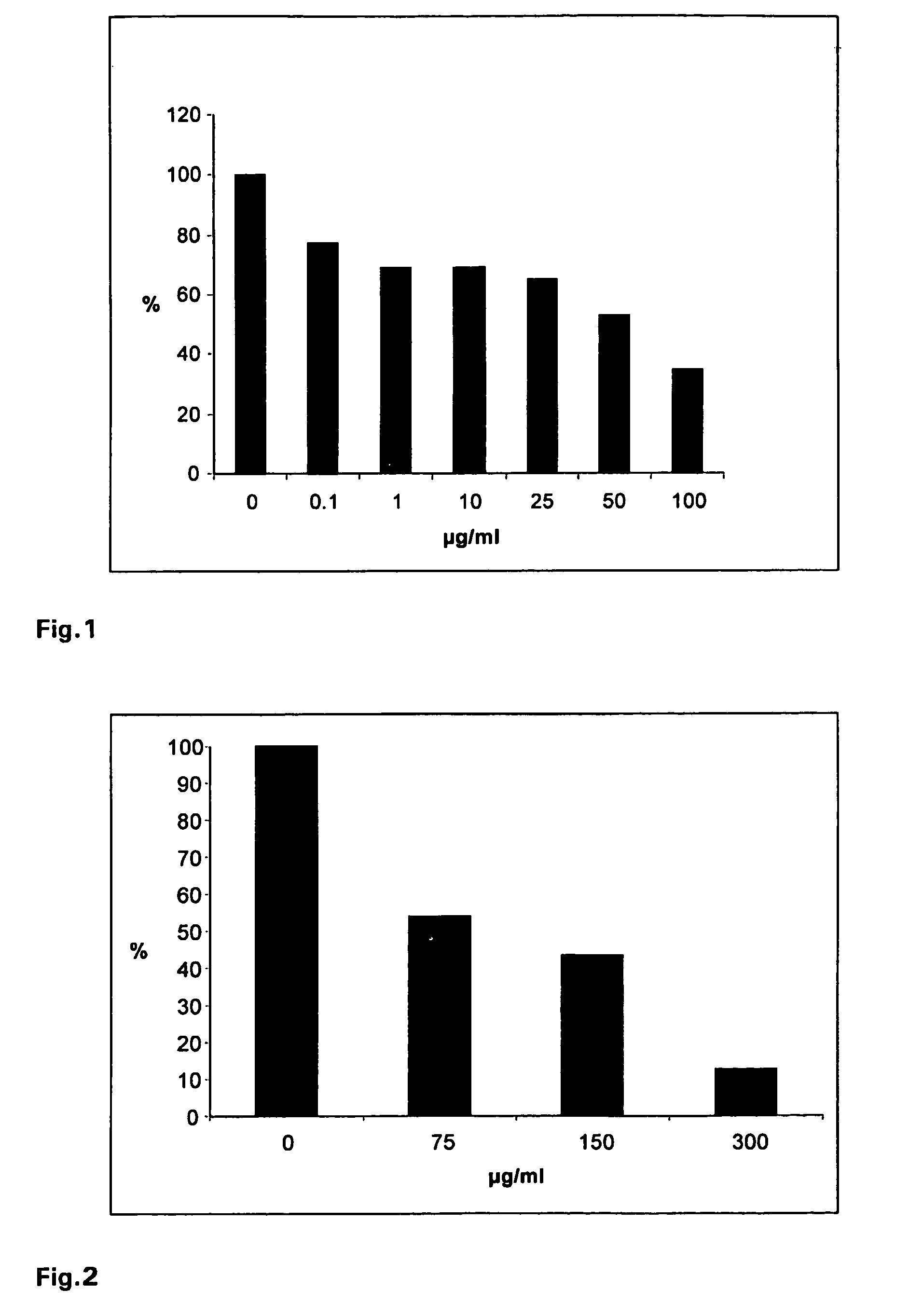
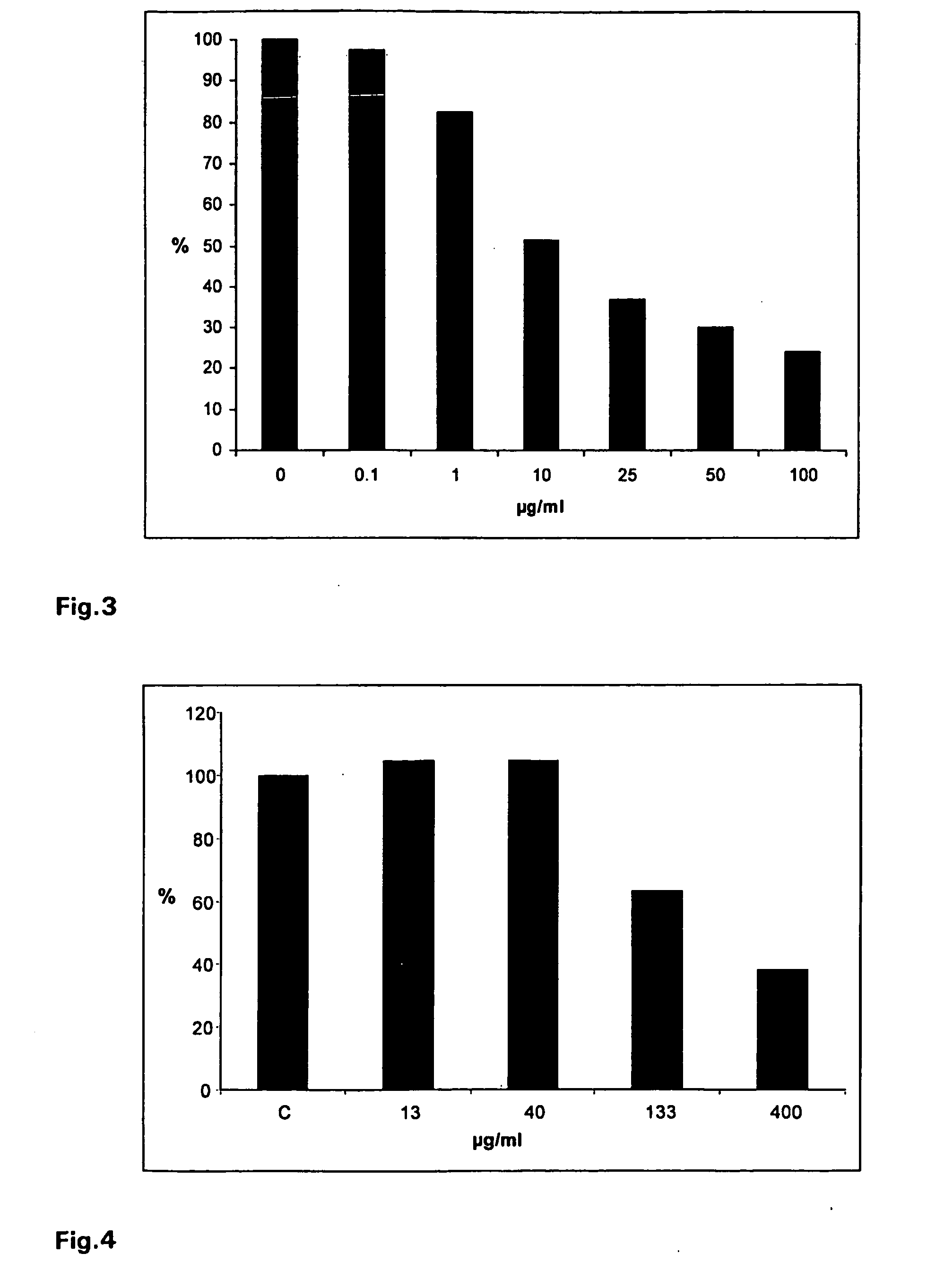
[0112]Virus suspensions containing 60-80 pfu of influenza virus A / Chile / 1 / 93 H1N1 were mixed with a iota-carrageenan stock solution to final concentrations of 0.1, 1, 10, 25, 50 or 100 μg / ml. Confluent monolayers of the canine kidney cell line MDCK in six well plates were infected with the virus suspensions for 60 min at 34° C. The infection inoculum was removed and cells were washed with PBS and agarose overlay containing 0.6% agarose was added. Plates were incubated at 36° C. in a humidified atmosphere of 5% CO2 in air. 48-60 h after infection the agarose overlay was removed, cells were stained with crystal violet stain and visible plaques were counted. The percentage of plaque formation relative to the infected control (without iota-carrageenan treatment) was determined for each iota-carrageenan concentration.
[0113]As shown in FIG. 1, it was found that iota-carrageenan inhibi...
example 2
Effect of Different Concentrations of Iota-Carrageenan on Influenza A Virus Plaque Formation in MDCK Cells
[0114]Virus suspensions containing 60-80 pfu of influenza virus A / Aichi2 / 68H3N2 were mixed with a iota-carrageenan stock solution to final concentrations of 75, 150 or 300 μg / ml. Confluent monolayers of the canine kidney cell line MDCK in six well plates were infected with the virus suspensions for 60 min at 34° C. The infection inoculum was removed and cells were washed with PBS and agarose overlay containing 0.6% agarose was added. Plates were incubated at 37° C. in a humidified atmosphere of 5% CO2 in air. 48-60 h after infection the agarose overlay was removed, cells were stained with crystal violet stain and visible plaques were counted. The percentage of plaque formation relative to the infected control (without iota-carrageenan) was determined for each iota-carrageenan concentration.
[0115]As shown in FIG. 2, it was found that iota-carrageenan inhibits, in a dose dependent...
example 3
Effect of Different Concentrations of Iota-Carrageenan on Parainfluenza Virus 3 Plaque Formation in Hep-2 Cells
[0116]Virus suspensions containing 60-80 pfu of parainfluenza virus 3 were mixed with a iota-carrageenan stock solution to final concentrations of 0.1, 1, 10, 25, 50 and 100 μg / ml. The mixture was incubated for 1 h at 34° C. Confluent monolayers of Hep-2 cells in six well plates were infected with the virus suspensions for 60 minutes a 34° C. The infection inoculum was removed and cells were washed with PBS and agarose overlay containing 0.6% agarose was added. The trays were incubated in a humidified, 5% CO2 atmosphere. 48-60 h after infection the agraose overlay was removed, cells were stained with crystalviolett stain and visible plaques were counted. The percentage of plaque formation relative to the infected control (without iota-carrageenan) plates was determined for each iota-carrageenan concentration.
[0117]As shown in FIG. 3, it was found that iota-carrageenan inhib...
PUM
| Property | Measurement | Unit |
|---|---|---|
| average molecular weight | aaaaa | aaaaa |
| average molecular weight | aaaaa | aaaaa |
| molecular weights | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com