Methods and materials for producing immune responses against polypeptides involved in antibiotic resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Plasmids Containing Codon-optimized Antigens
[0029]Codon-optimized sequences for Mtb antigens are obtained from Genscript Corporation (Piscataway, N.J.) or generated using molecular cloning techniques. These sequences are codon-optimized for expression in mammalian cells for use as gene-based vaccines. The following Mtb genes that are expressed by H37Rv, MDR Mtb, or XDR Mtb or those listed in Table 1 that are expressed by H37Rv, MDR Mtb, or XDR Mtb are synthesized and cloned into the pShuttle-CMV plasmid:
[0030]Ag85a: Positive control protective antigen.
[0031]whiB7: Inducer of expression of a regulon of Mtb genes involved in antibiotic resistance (including tap, RV1473, and erm (Morris et al., Proc. Nat'l. Acad. Sci. USA, 102(34):12200-12205 (2005)).
[0032]tap: Drug efflux pump conferring low level resistance to aminoglyosides and tetracycline.
[0033]RV1473: Putative macrolide transporter induced by whiB7 (Morris et al., Proc. Nat'l. Acad. Sci. USA, 102(34):12200-12205 (2005)).
erm: Conf...
example 2
Adenovirus Vectors Containing Codon-Optimized Antigens
[0035]Ad5 vectors are used to generate gene-based vaccines, which are used as an effective vaccine delivery vehicle in mice. Any appropriate vaccine carrier including Bacillus Calmette-Guerin (BCG) or vaccinia is used as a vaccine delivery vehicle in humans. In some cases, the recombinant polypeptides are delivered directly to the mammal (e.g., a human).
[0036]Once the pShuttle-CMV vectors are obtained, they are recombined into the Ad5 genome in bacteria and are used to generate CsCl-purified Ad5 vaccines.
example 3
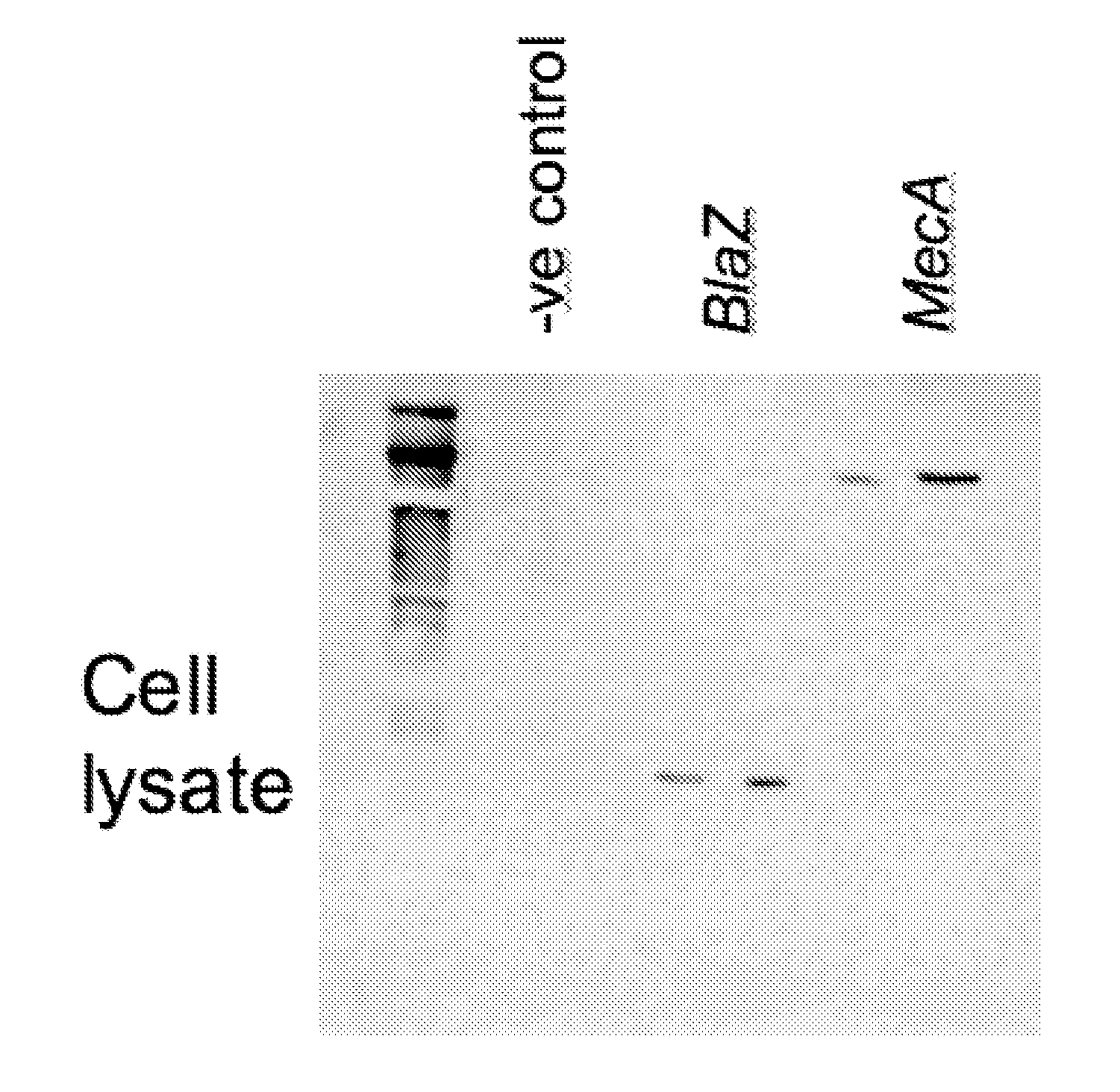
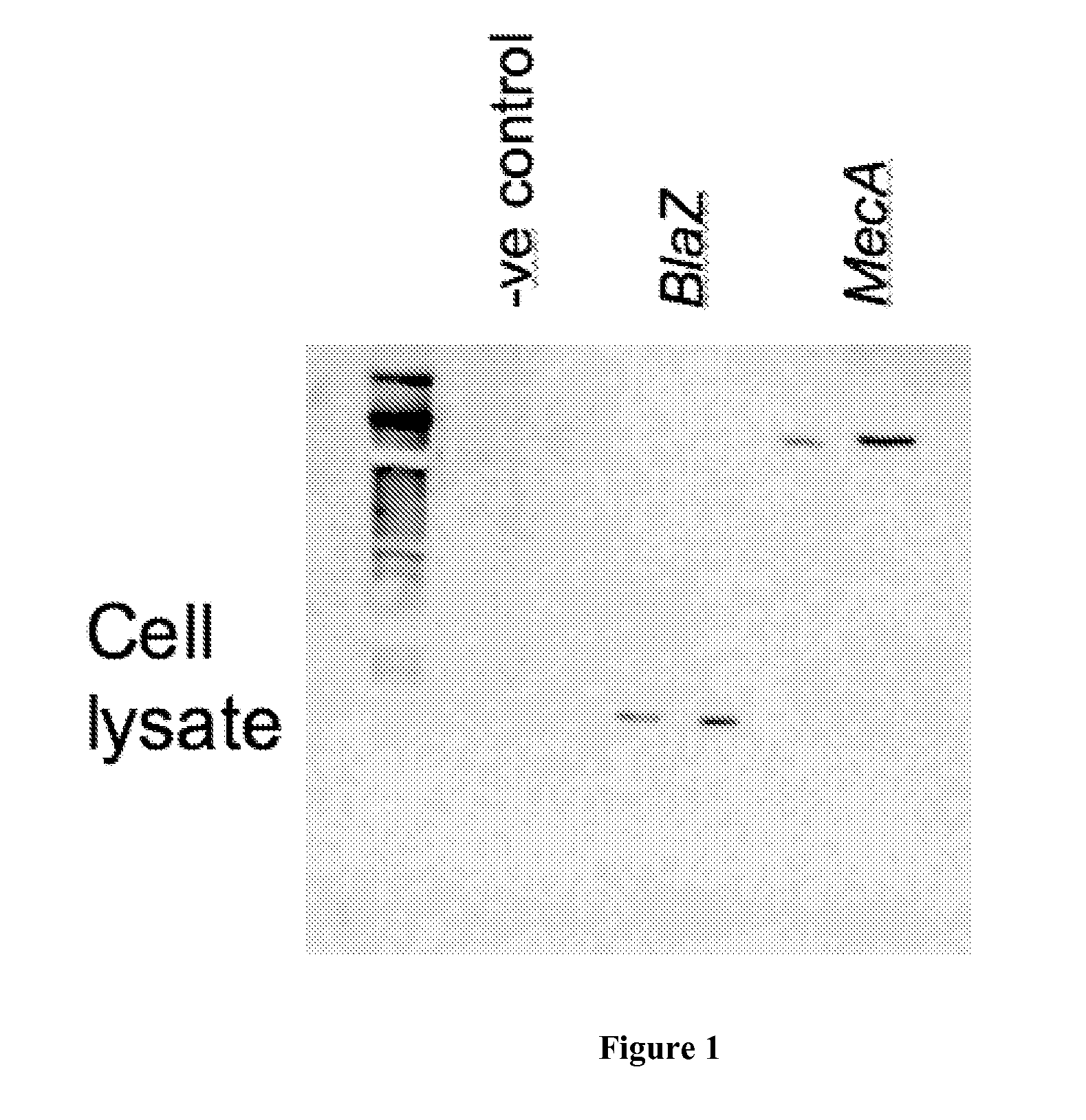
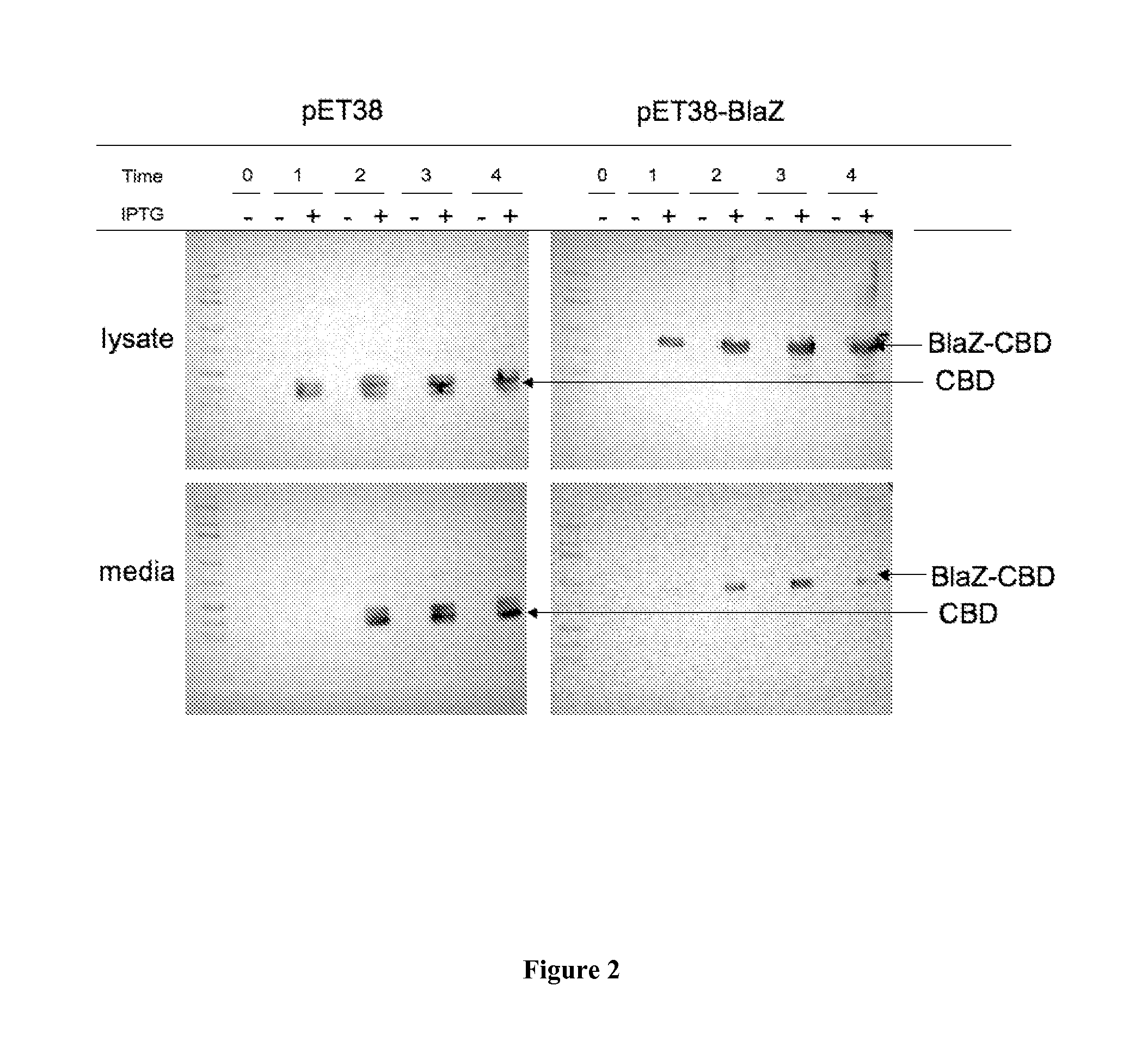
Codon-Optimized Gene for S. aureus BlaZ
[0037]The following codon-optimized nucleic acid sequence was generated to encode an S. aureus BlaZ polypeptide: AAGGAGCTGAACGACCTGGAGAAGAAGTACAACGCCC-ACATCGGCGTGTACGCCCTGGACACCAAGAGCGGCAAGGAGGTGAAGTTCAACA GCGACAAGCGCTTCGCCTACGCCAGCACCAGCAAGGCCATCAACAGCGCCATCC TGCTGGAGCAGGTGCCCTACAACAAGCTGAACAAGAAGGTGCACATCAACAAGG ACGACATCGTGGCCTACAGCCCCATCCTGGAGAAGTACGTGGGCAAGGACATCA CCCTGAAGGCCCTGATCGAGGCCAGCATGACCTACAGCGACAACACCGCCAACA ACAAGATCATCAAGGAGATCGGCGGCATCAAGAAGGTGAAGCAGCGCCTGAAG GAGCTGGGCGACAAGGTGACCAACCCCGTGCGCTACGAGATCGAGCTGAACTAC TACAGCCCCAAGAGCAAGAAGGACACCAGCACCCCCGCCGCCTTCGGCAAGACC CTGAACAAGCTGATCGCCAACGGCAAGCTGAGCAAGGAGAACAAGAAGTTCCTG CTGGACCTGATGCTGAACAACAAGAGCGGCGACACCCTGATCAAGGACGGCGTG CCCAAGGACTACAAGGTGGCCGACAAGAGCGGCCAGGCCATCACCTACGCCAGC CGCAACGACGTGGCCTTCGTGTACCCCAAGGGCCAGAGCGAGCCCATCGTGCTG GTGATCTTCACCAACAAGGACAACAAGAGCGACAAGCCCAACGACAAGCTGATC AGCGAGACCGCCAAGAGCGTGATGAAGGAGTTC (SEQ ID NO:1). The amino acid sequence encoded by SEQ ID NO...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap