Transscleral delivery
a transscleral and ocular technology, applied in the direction of biocide, drug composition, extracellular fluid disorder, etc., can solve the problems of fibrovascular scarring of the macular region, no treatment, and current treatment that is not optimal,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Inhibition of Laser Induced CNV by Rapamycin
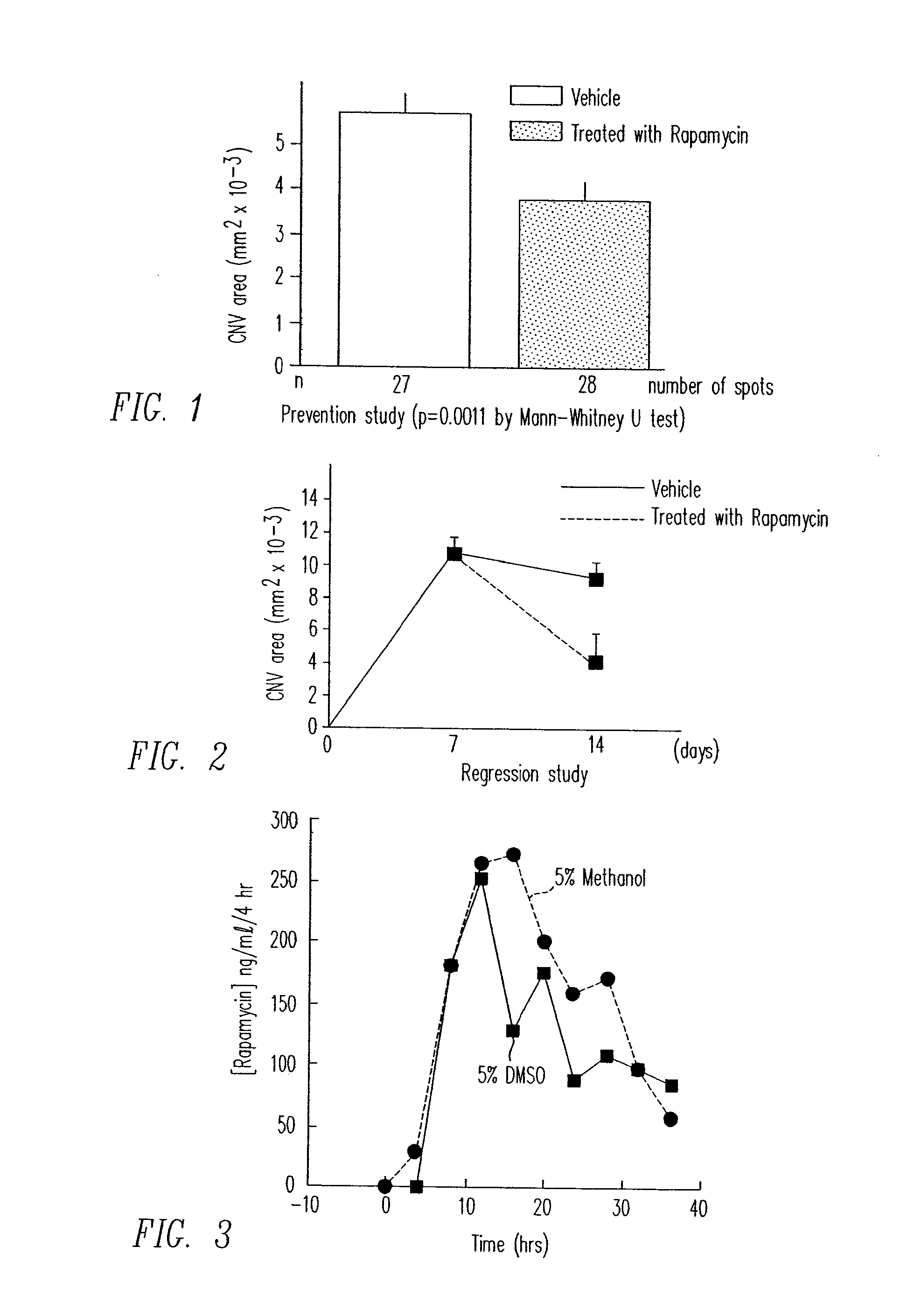
[0196]CNV may be induced by rupturing Bruch's membrane with laser. Fifteen mice were given daily periocular injections of 5 micrograms of rapamycin in corn oil vehicle in one eye. After two days of treatment, Bruch's membrane was ruptured by laser at three sites in each eye. The fellow eye served as control and was treated with periocular injections of corn oil only. Two weeks after laser rupture, 10 mice were perfused with fluorescein-labeled dextran and CNV areas were measured in each eye on choroidal flat mounts. The remaining mice had retinas and RPE / choroid dissected and stored at −70° C. for tissue therapeutic agents level measurement.
[0197]As depicted in FIG. 1, rapamycin treatment led to a statistically significant reduction in CNV size versus vehicle alone (p=0.0011 by Mann-Whitney U test). The mean CNV area for rapamycin treated eyes was 0.00381 mm2 with a standard deviation of 0.00197 and the vehicle eyes showed a CNV area of 0....
example 2
Reversal of Laser Induced CNV by Rapamycin
[0200]Fifteen mice had Bruch's membrane ruptured with laser photocoagulation at 3 locations in each eye. After one week, 5 mice were perfused with fluorescein-labeled dextran and the baseline area of CNV was measured in each eye. At that point, the remaining mice were started on daily periocular injections of 5 μl of corn oil containing 5 μg of rapamycin in one eye and corn oil alone in the fellow eye. After one more week, the mice were perfused with fluorescein-labeled dextran and CNV areas were measured in each eye on choroidal flat mounts. The corn oil solution used is approximately a saturated solution. It is expected that lower doses will be used to deliver therapeutically effective amounts of rapamycin.
[0201]As depicted in FIG. 2, rapamycin treated eyes showed a substantial reduction in CNV area. Baseline lesion CNV area was 0.0105 mm2 with a standard deviation of 0.0037. Untreated eyes had a CNV area of 0.0093 mm2 with a standard devi...
example 3
In-vitro Determination of Flux and Scleral Permeability for Transscleral Rapamycin Delivery
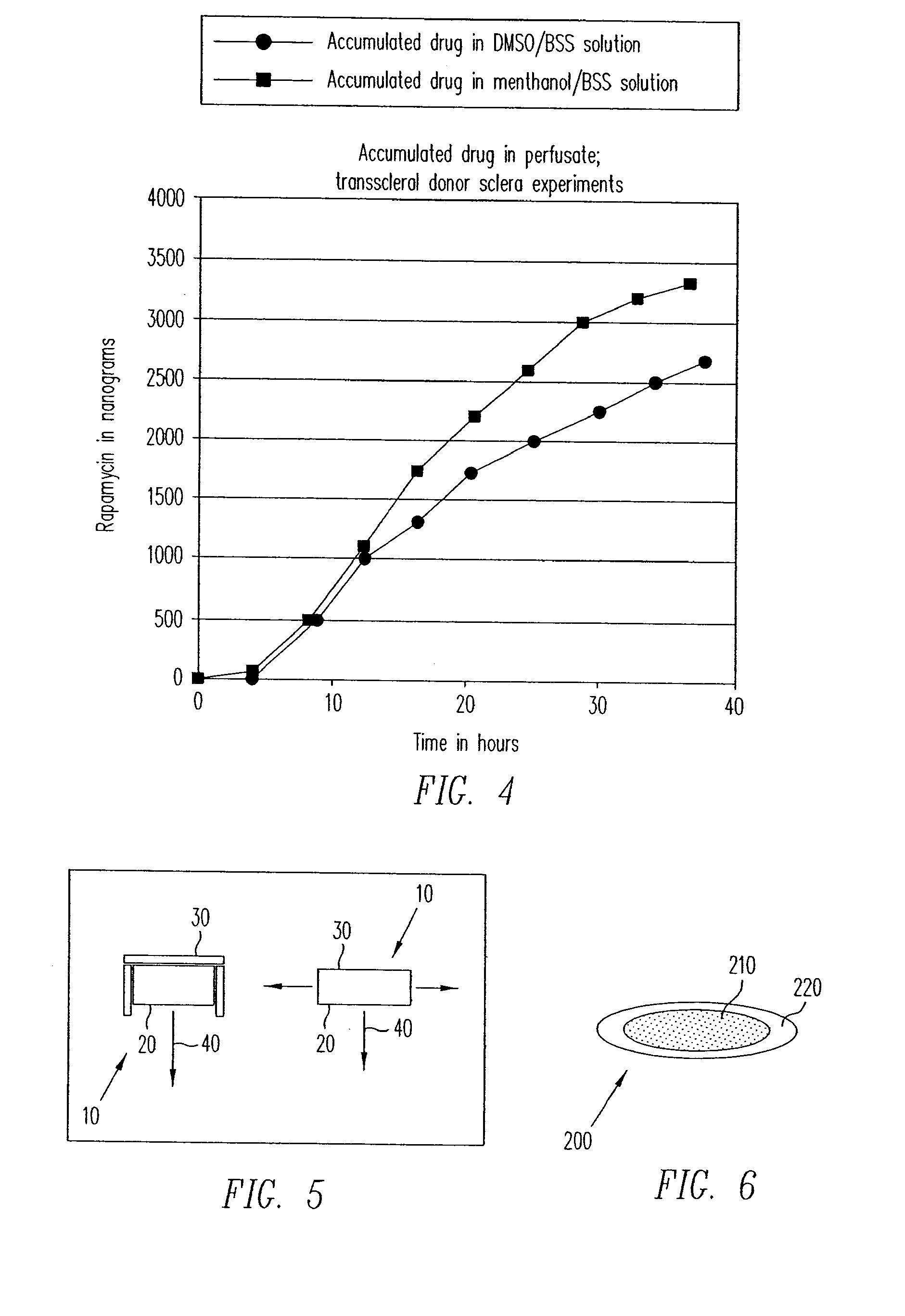
[0203]A two chamber Ussing type permeability apparatus was used to demonstrate delivery of rapamycin across a human sclera. Prior to testing of sclera, loss of therapeutic agent to the glass walls of the experimental apparatus was evaluated. For the evaluation of loss of drug to the experimental apparatus, the uveal and orbital chambers were exposed to a 2 μg / mL rapamycin solution in balanced salt solution. There was small but significant loss of rapamycin to the apparatus when it was exposed to the 2 μg / mL solution of rapamycin in balanced salt solution.
[0204]Transscleral Rapamycin Delivery—DMSO Solution
[0205]A 7 mm disk of fresh donor human sclera was used to separate the two chambers of the Ussing permeability apparatus. The two sides of the sclera were denoted “orbital” to represent the outer surface and “uveal” to represent the internal surface. DMSO and methanol dissolves rapamycin readi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com