STABILIZATION OF IMMUNOGLOBULINS AND OTHER PROTEINS THROUGH AQUEOUS FORMULATIONS WITH SODIUM CHLORIDE AT WEAK ACIDIC TO NEUTRAL ph
a technology of sodium chloride and immunoglobulin, which is applied in the direction of antibody medical ingredients, inorganic non-active ingredients, extracellular fluid disorders, etc., to achieve the effects of stably formulating labile proteins, stably formulating labile therapeutic proteins, and simple self-administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0642]To determine the role pH and salt concentration have on a plasma-derived 20% IgG composition, a two year stability study was conducted. This study revealed that the inclusion of sodium chloride and / or the formulation at neutral to mildly acid pH imparted a stabilizing effect on the 20% IgG composition.
[0643]Briefly, two IgG compositions prepared from pooled plasma according to the Gammagard SD process outlined in Teschner et al. (Vox Sang. 2007 January; 92(1):42-55) were concentrated to a final protein concentration of 20%. These preparations were then divided into several samples which were differentially formulated at pHs 6.5, 7.0, or 7.5 with and without 50 mM sodium chloride. The aqueous formulations were then stored at between 28° C. and 32° C. for 24 months. After the two year incubation period, the molecular size distributions of the IgG in the various formulations were investigated by high performance size exclusion chromatography (HP-SEC), the results of which are pro...
example 2
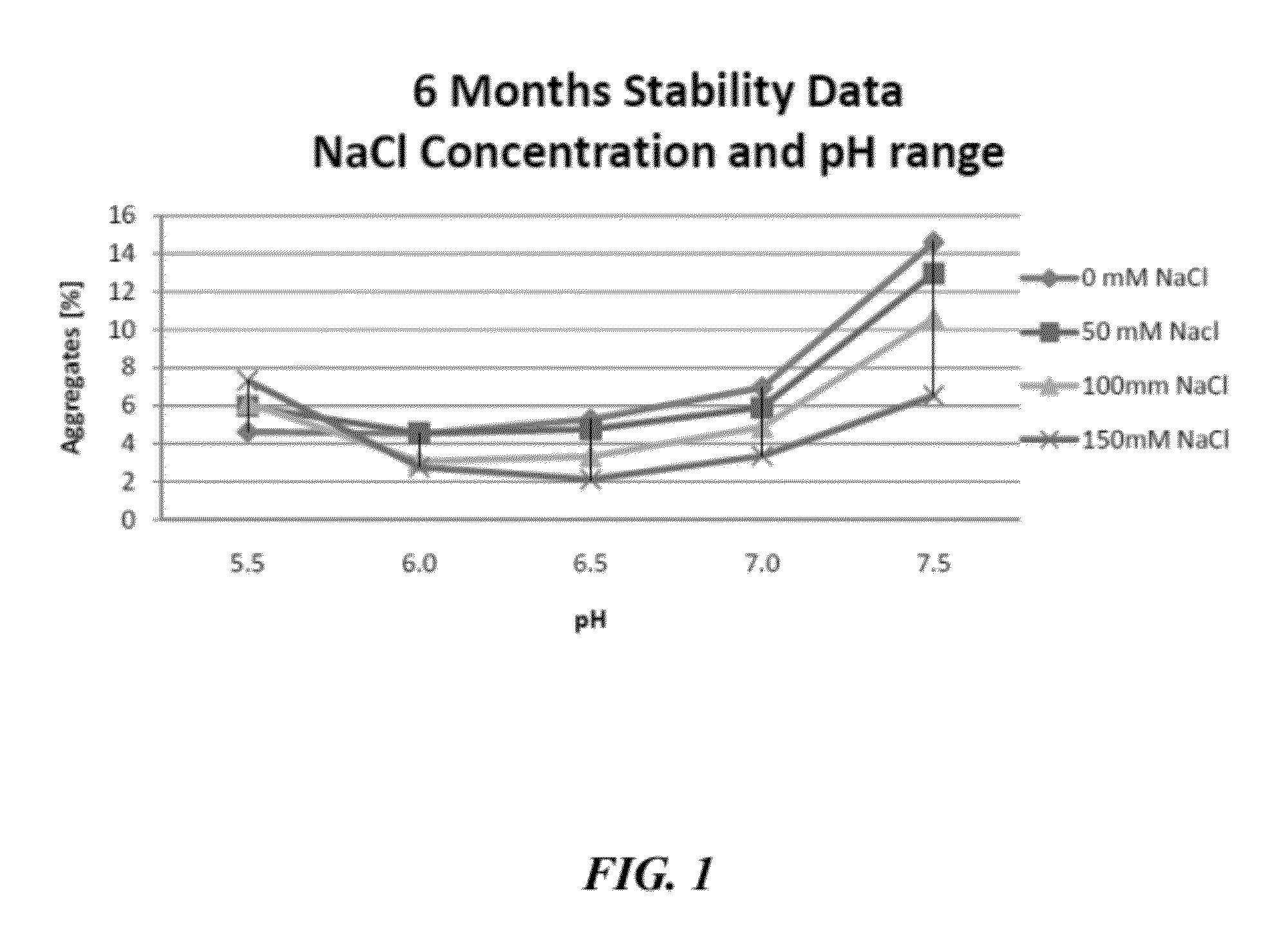
[0645]To further characterize the stabilizing effect that sodium chloride has on IgG compositions formulated at mildly acid to neutral pH, an accelerated stability study was performed. For the accelerated study, elevated temperatures (38° C. to 42° C.) were used to simulate longer time periods at room temperature (20° C. to 25° C.). Briefly, a 20% IgG composition, prepared as in Example 1, was divided into samples that were formulated with increasing salt concentrations (0 mM, 50 mM, 100 mM, and 150 mM) at mildly acid to neutral pHs (pH 5.5, 6.0, 6.5, 7.0, and 7.5). The aqueous formulations were then stored at between 38° C. and 42° C. for 6 months. After the 6 month incubation period, the molecular size distributions of the IgG in the various formulations were investigated by high performance size exclusion chromatography (HP-SEC). The percentage of IgG aggregates present in the various formulations is shown in FIG. 1.
[0646]As seen in FIG. 1, the stability of the immunoglobulin pre...
example 3
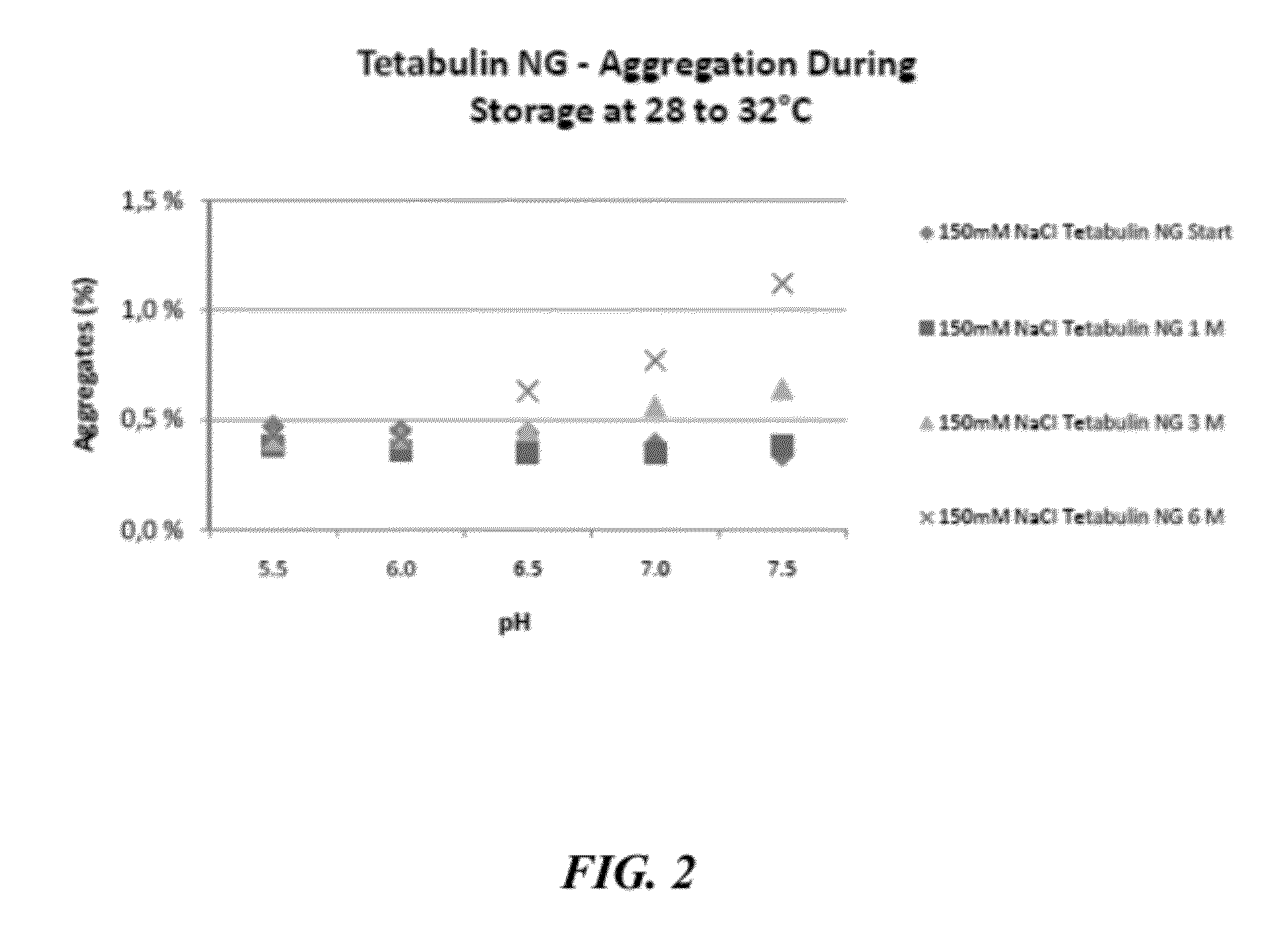
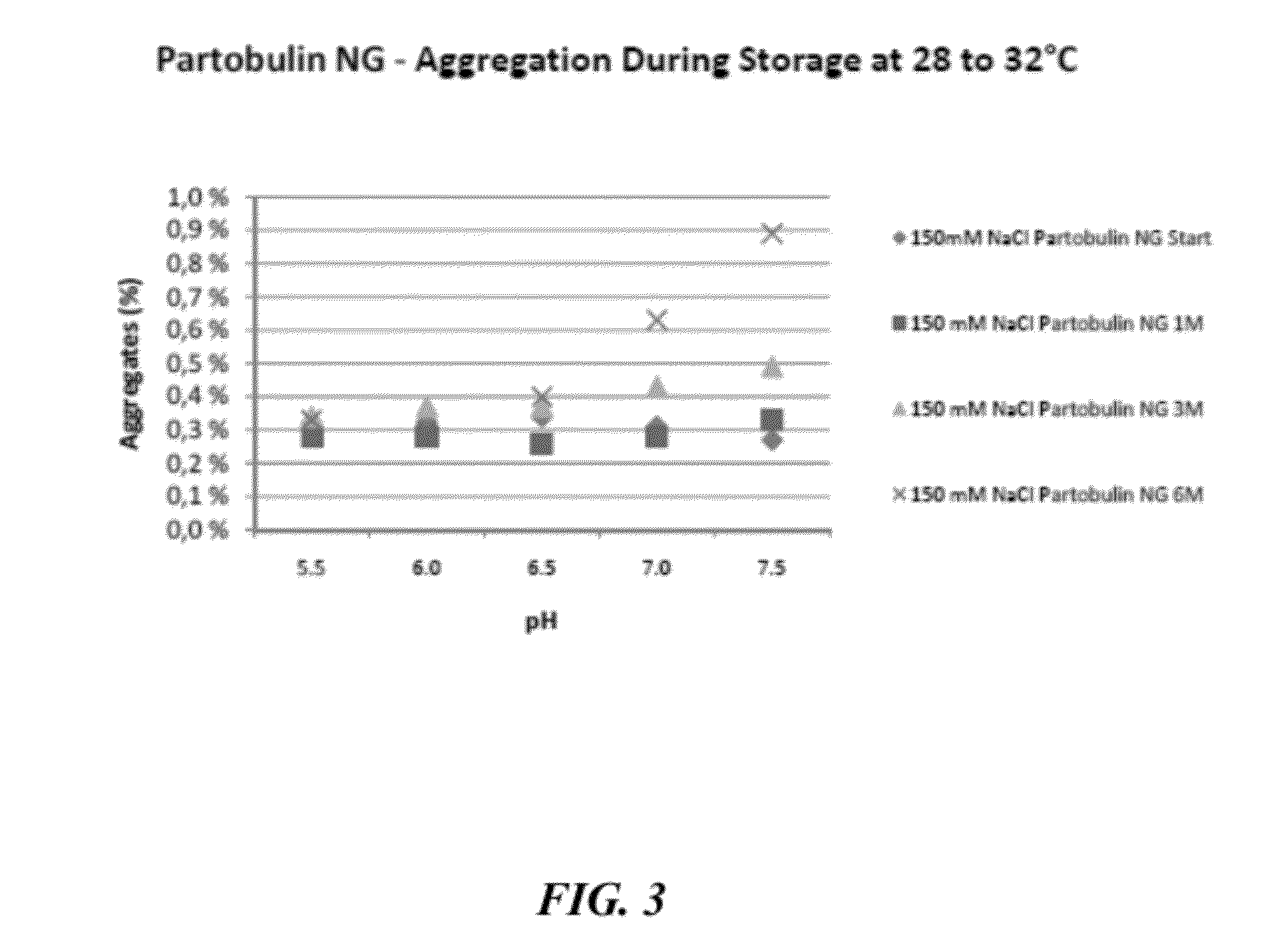
[0647]To evaluate the stabilizing effects of sodium chloride on other immunoglobulin preparations formulated at mildly acidic to neutral pH, preparations of Partobulin® NG (Baxter Biosciences) and Tetabulin® NG (Baxter Biosciences) were formulated accordingly and tested for stability, via aggregation formation, and activity, via anti-antigen potency, over a 6 month time frame. Partobulin® is a plasma-derived human anti-D antigen immunoglobulin preparation used for antenatal anti-D prophylaxis in Rh(D) negative pregnant women carrying Rh(D) positive fetuses, as well as for the treatment of Rh(D) negative persons after incompatible transfusions of Rh(D) positive blood or erythrocyte concentrate. Tetabulin® is a plasma-derived human tetanus immunoglobulin used for post-tetanus exposure prophylaxis and therapy of clinically manifest tetanus. Both Partobulin® and Tetabulin® are typically formulated at between 100 g / L and 170 g / L human protein (of which at least 90% are immunoglobulin G) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com