Nanometer size chemical modified materials and uses
a chemical modification and nanometer technology, applied in the field of nanometer size chemical modification materials and uses, can solve the problems of high surface coverage and high hydrolytic stability of stationary phases, remain virtually unexplored in the field of separation, use of poss grafted, etc., to improve hydrolytic stability, increase hydrophobicity, and high ligand coverage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
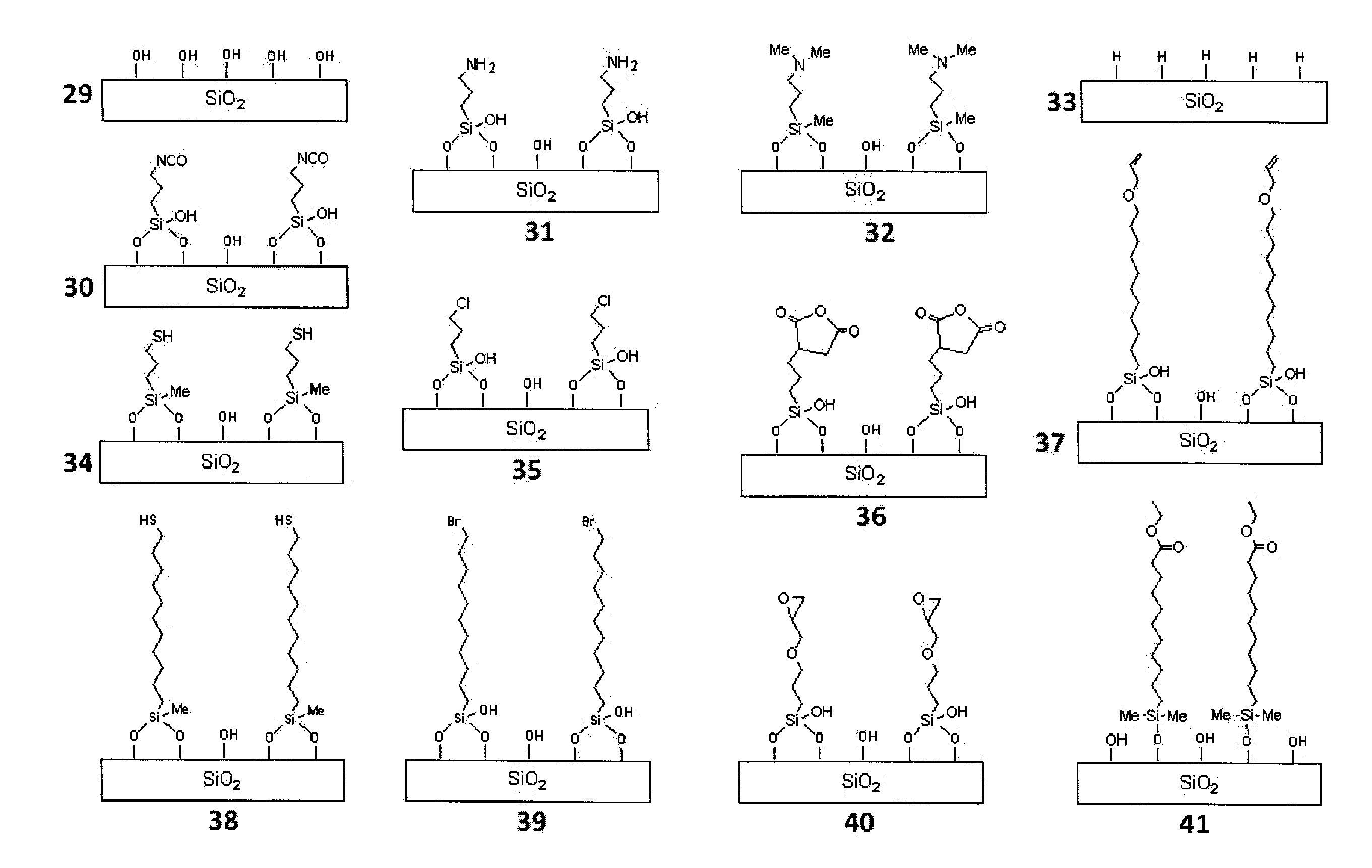
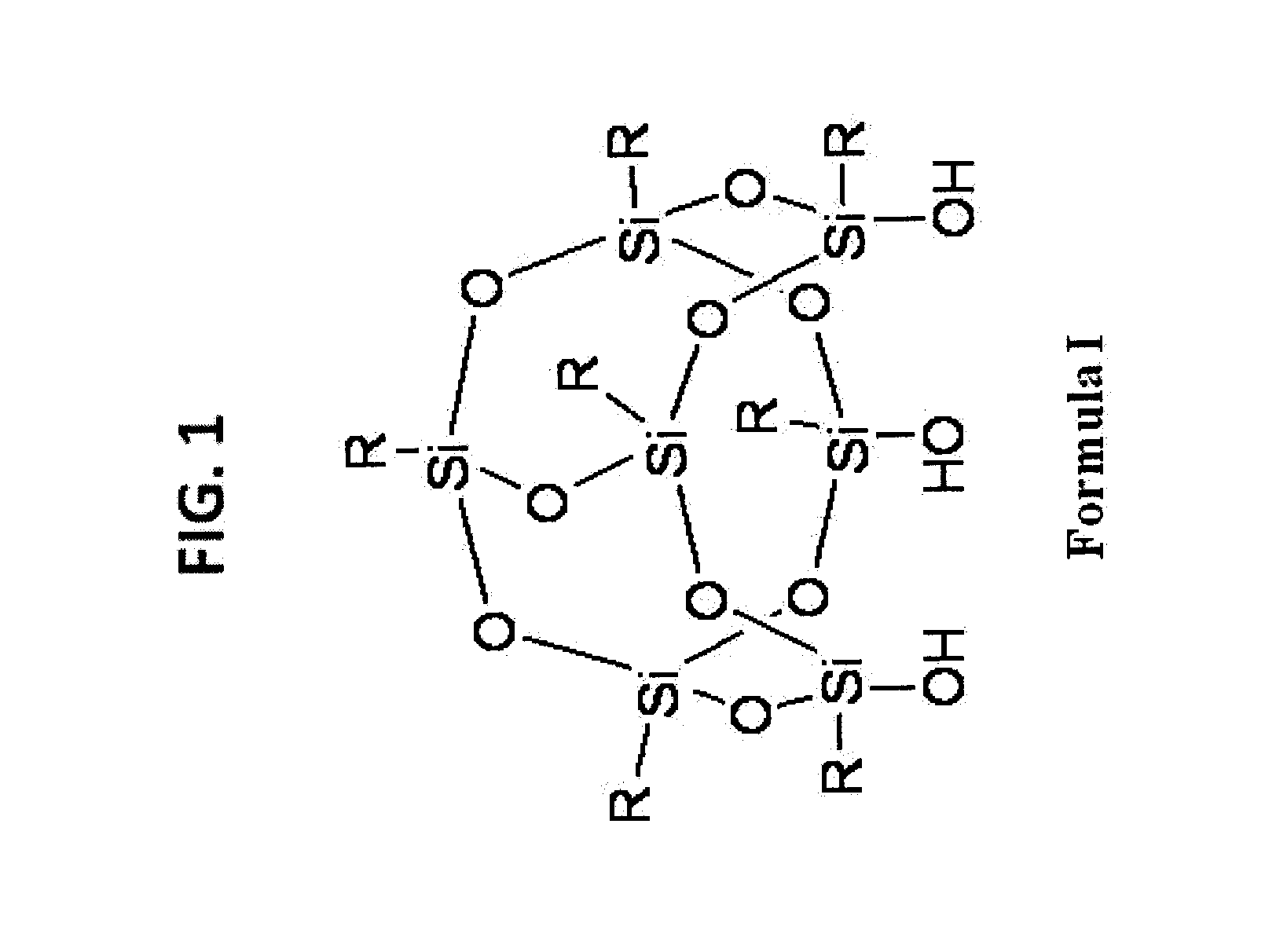
[0171]1) Functionalization-General Conditions
General Synthetic Procedure of Making T7-POSS Bonded Phases 42-49
[0172]A selected T7R7(OH)3-POSS compound is dissolved in an appropriate high boiling point solvent in a round bottom flask. A suitable quantity of silica gel is dispersed in this solution. After reflux for 24 to 96 hours, the reaction mixture is filtered. The cake is then washed with sufficient quantity of a suitable solvent in which the POSS compound can be dissolved. The resulting material is dried in a vacuum oven at 60° C. for 12 hours.
[0173]Alternatively, a T7R7(OH)3-POSS compound is dissolved in an appropriate low boiling point solvent in a round bottom flask. Then a suitable quantity of silica gel is dispersed in this solution. After carefully removing all volatiles on a rotovap under reduced pressure, the resulting substance is heated at 160° C. for 12 hours. Then the reaction mixture is filtered and the cake is washed with sufficient quantity of a suitable solvent i...
example 2
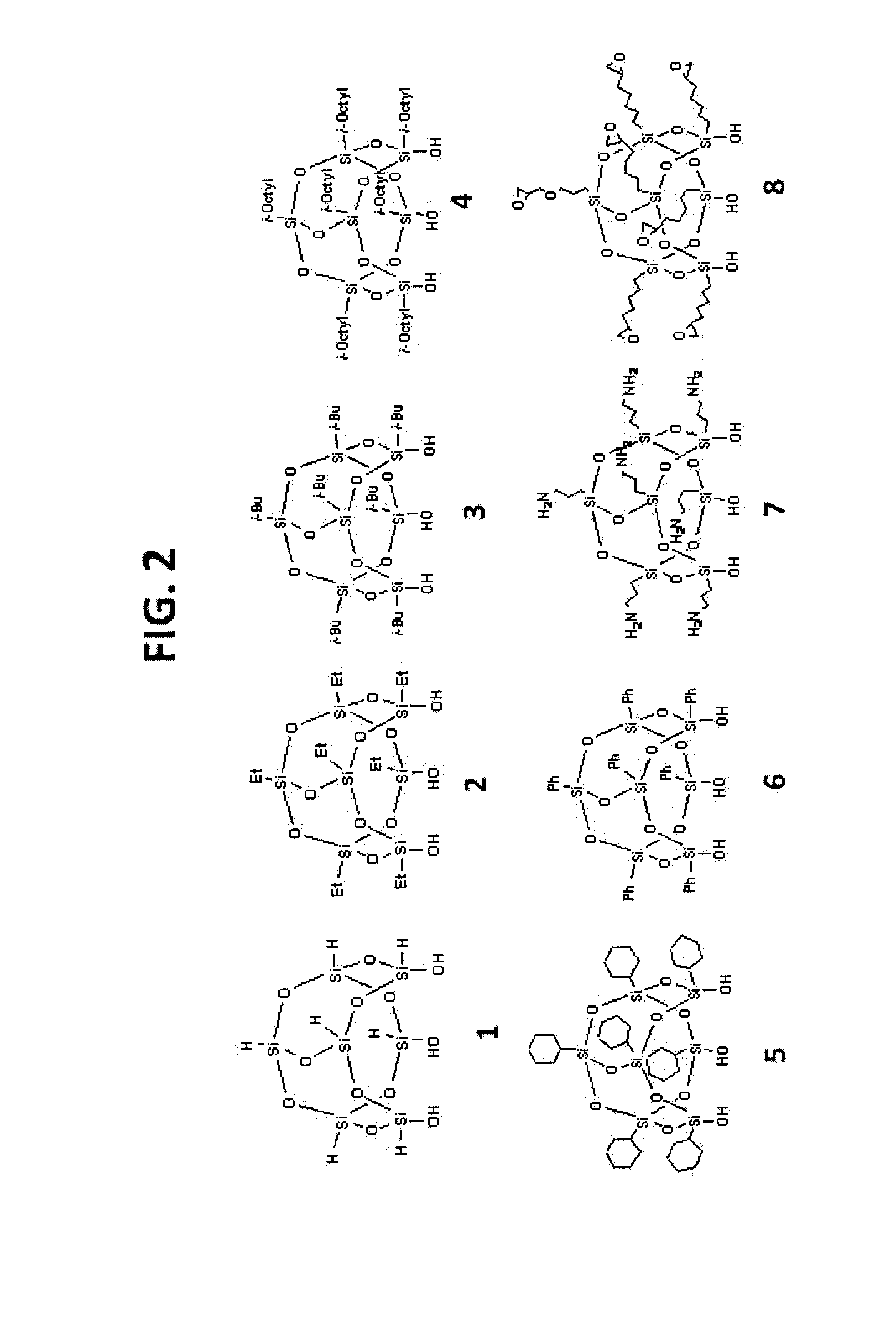
Synthesis of iso-Butyl-T7 POSS Phase (43)
[0177]10 g Trisilanolisobutyl POSS (3) is dissolved in 100 mL of decane in a 200-mL round bottom flask. 10 g of raw silica gel (5.0 μm; specific surface area, 300 m2 / g; mean pore size, 120 Å; pore volume, 1.00 mL / g) is dispersed in this solution. After reflux for 72 hours, the reaction mixture is filtered. The cake is then washed with sufficient quantity of heptane. The resulting material is dried in a vacuum oven at 60° C. for 12 hours. The elemental analysis yields a carbon content of 7.01%, which corresponds to a ligand density of 3.88 μmol / m2.
example 3
Synthesis of iso-Octyl-T7 POSS Phase (44)
[0178]10 g Trisilanolisobutyl POSS (4) is dissolved in 100 mL of decane in a 200-mL round bottom flask. 10 g of raw silica gel (5.0 μm; specific surface area, 300 m2 / g; mean pore size, 120 Å; pore volume, 1.00 mL / g) is dispersed in this solution. After reflux for 72 hours, the reaction mixture is filtered. The cake is then washed with sufficient quantity of heptane. The resulting material is dried in a vacuum oven at 60° C. for 12 hours. The elemental analysis yields a carbon content of 9.37%, which corresponds to a ligand density of 3.85 μmol / m2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| median particle size | aaaaa | aaaaa |
| median particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com