Cytometry system with quantum cascade laser source, acoustic detector, and micro-fluidic cell handling system configured for inspection of individual cells
a microfluidic cell and laser source technology, applied in the field of cytometry system with quantum cascade laser source, microfluidic cell handling system configured for inspection of individual cells, can solve the problems of low accuracy and safety concerns, no safe and accurate method for cell sorting, and the facs process has been shown
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
)
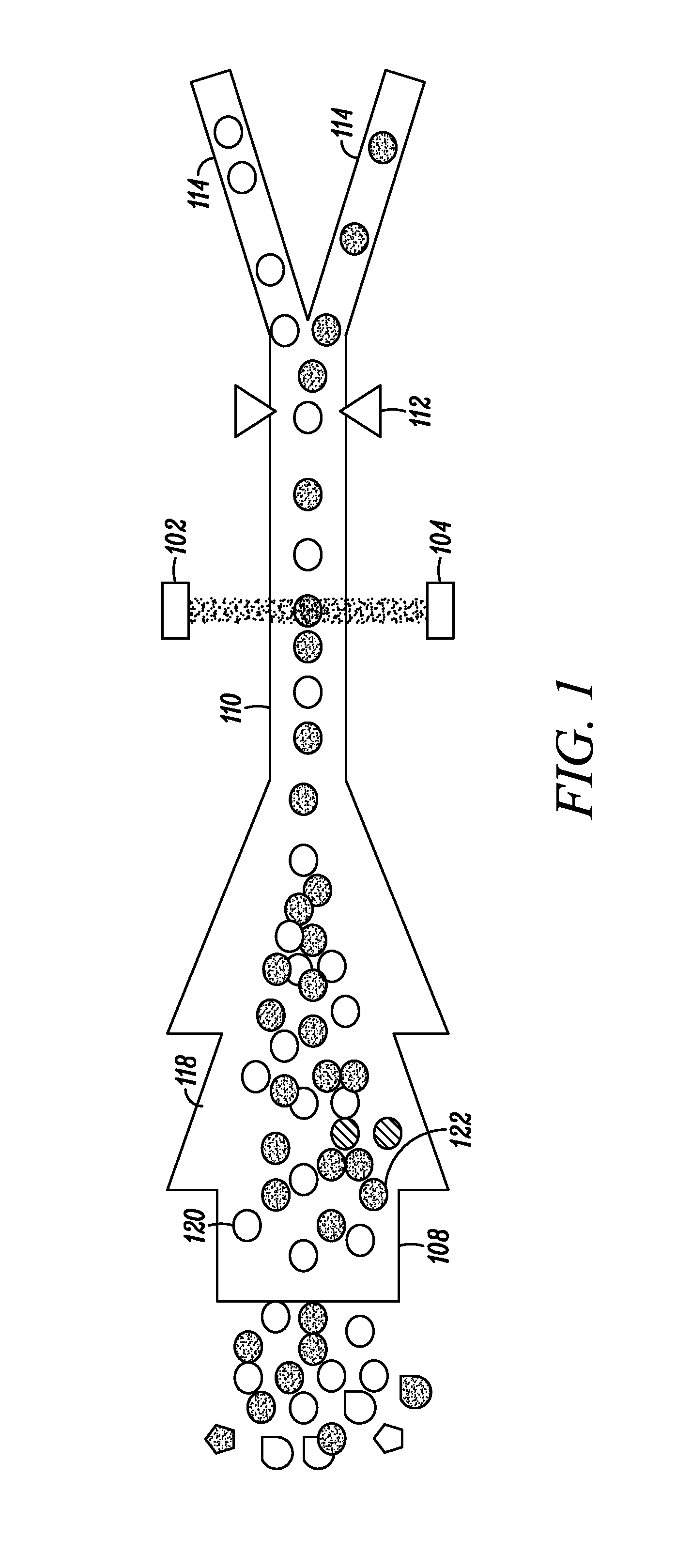
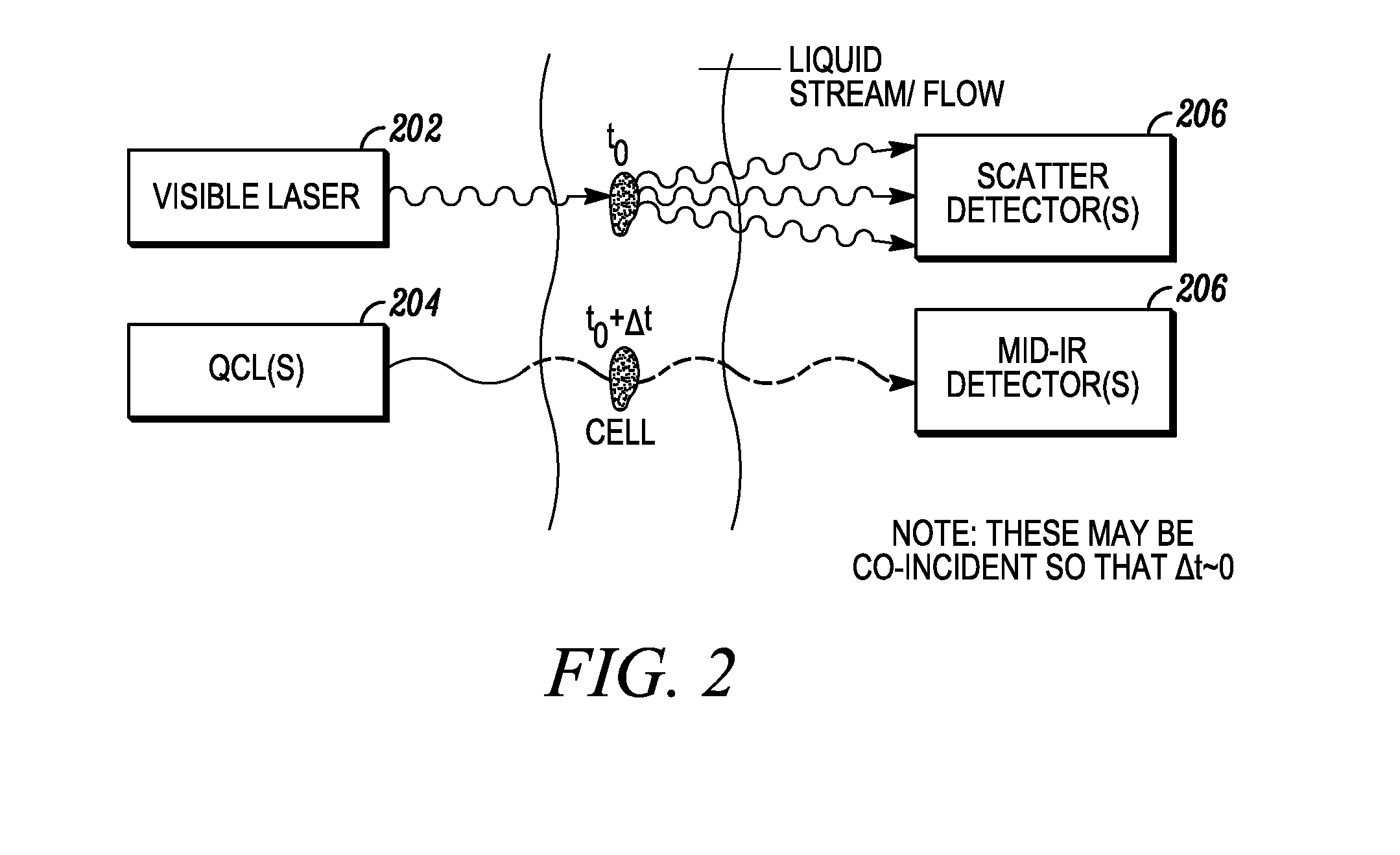
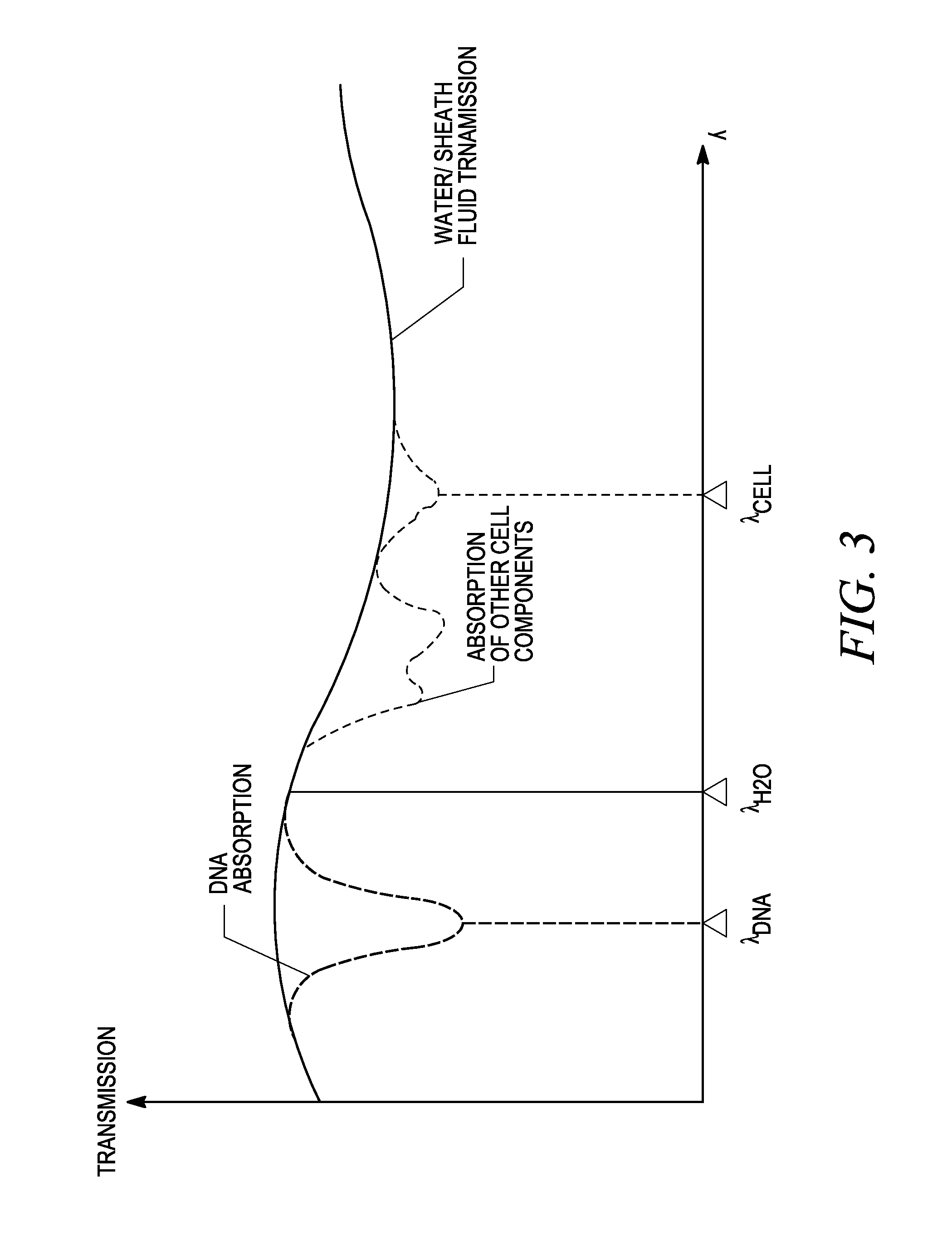
[0099]The invention described herein presents a novel approach to cellular measurements based on mid-infrared absorption measurements. Mid-IR quantum cascade lasers (QCLs) have the potential to focus sufficient energy onto a single cell to make an accurate, high-speed measurement. QCLs have recently been commercialized by multiple vendors. They are built using the same processes and packaging as high-volume telecom lasers. QCLs offer several advantages over traditional mid-IR sources such as delivering very high spectral power density, delivering very high spatial or angular power density, among other advantages. This allows QCLs to put 10,000,000× more effective mid-IR power onto a single cell than traditional mid-IR sources. This disclosure seeks to describe certain applications enabled by QCLs, such as label-free detection without dyes or labels that can alter or damage cells, measurements using mid-IR illumination 25× less energetic than that used in FACS, eliminating photon da...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com