Alpha-Amylases
a technology of alpha-amylase and beta-amylase, applied in the field of alpha-amylase variants, can solve the problem of disadvantage of calcium requiremen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Hybrids
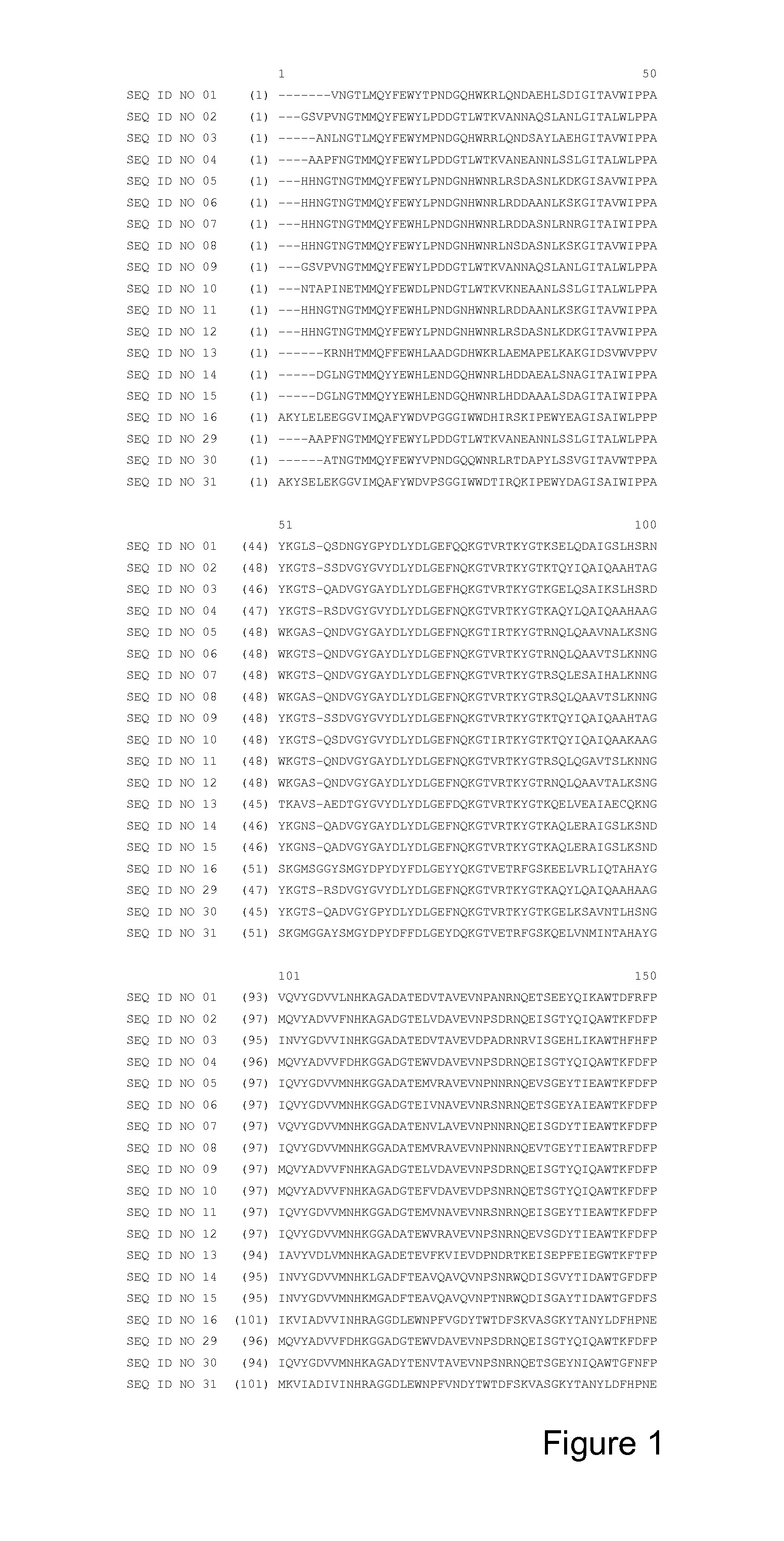
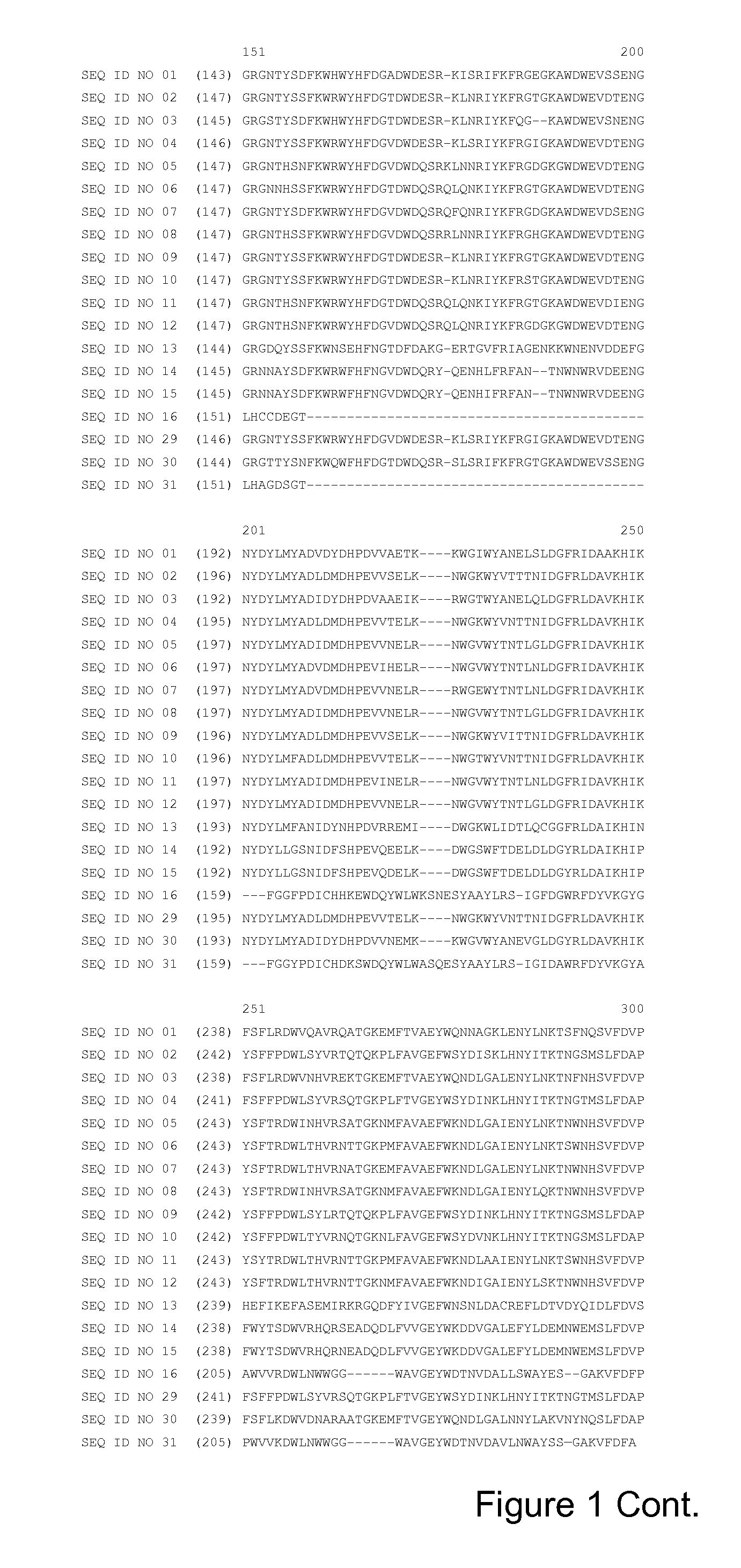
[0438]The following hybrids of the calcium-sensitive alpha-amylase having the sequence shown in SEQ ID NO: 7 and the calcium-insensitive alpha-amylase having the sequence shown in SEQ ID NO: 13 were prepared.
Hybrid 1: the amino acid residues 106-215 of SEQ ID NO: 7 were removed and replaced with the amino acid residues 103-211 of SEQ ID NO: 13, which results in SEQ ID NO: 17, and the following alterations were introduced: E182*, N183*, E188W, N189E and D192T (using SEQ ID NO: 17 numbering), which correspond to E181*, N182*, E187W, N188E and D191T using SEQ ID NO: 27 numbering. The sequence of this hybrid is shown in SEQ ID NO: 18.
Hybrid 2: the amino acid residues 106-214 of SEQ ID NO: 7 were removed and replaced with the amino acid residues 103-210 of SEQ ID NO: 13, which results in SEQ ID NO: 19, and the following alterations were introduced: E182*, N183*, E188W, N189E and D192T (using SEQ ID NO: 19 numbering), which correspond to E181*, N182*, E187W, N188E an...
example 2
Stability in the Presence of Chelator
[0439]Enzyme samples were incubated in buffer pH 8.0 (50 mM EPPS, 0.01% TRITON®X100, pH 8.0) with 1.5% final concentration of DTPA at 49° C. for 1 hour and reference samples were incubated at 4° C. for 1 hour. In addition, enzyme samples were incubated in buffer pH 10.0 (50 mM EPPS, 0.01% TRITON® X100, pH 10.0) with 1.5% final concentration of DTPA at 42° C. for 1 hour and reference samples were incubated at 4° C. for 1 hour.
[0440]For the determination of amylase stability in buffer pH 8 and pH 10 with DTPA the enzymes to be tested were adjusted to 0.25 and 0.5 mg enzyme protein / mL by diluting in 5 mM EPPS, 0.01% TRITON® X100, pH 8.0.
[0441]160 microliters stability buffer (50 mM EPPS, 0.01% TRITON® X100, 1.875% DTPA, pH 8.0 or pH 10.0) and 40 microliters of the amylase solution were transferred to a 96-well PCR microtiter plate in duplicate and the content was mixed for 1 minute. Final concentration of DTPA was 1.5% in each well. 20 microliters f...
example 3
Additional Alpha-Amylases
[0444]The following alpha-amylases were prepared:
Hybrid 4: the amino acid residues 106-212 of SEQ ID NO: 5 were removed and replaced with the amino acid residues 103-208 of SEQ ID NO: 13, which results in SEQ ID NO: 23, and the following alterations were introduced: E182*, N183*, E188W, N189E and D192T (using SEQ ID NO: 23 numbering), which correspond to E181*, N182*, E187W, N188E and D191T in SEQ ID NO: 27 numbering. The sequence of this hybrid is shown in SEQ ID NO: 24.
Hybrid 5: the amino acid residues 106-212 of SEQ ID NO: 8 were removed and replaced with the amino acid residues 103-208 of SEQ ID NO: 13, which results in SEQ ID NO: 25, and the following alterations were introduced: E182*, N183*, E188W, N189E and D192T (using SEQ ID NO: 25 numbering), which correspond to E181*, N182*, E187W, N188E and D191T in SEQ ID NO: 27 numbering. The sequence of this hybrid is shown in SEQ ID NO: 26.
Hybrids 4 and 5 (SEQ ID NOS: 24 and 26), a variant of SEQ ID NO: 5 wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| nucleic acid | aaaaa | aaaaa |
| stability | aaaaa | aaaaa |
| crystal structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com