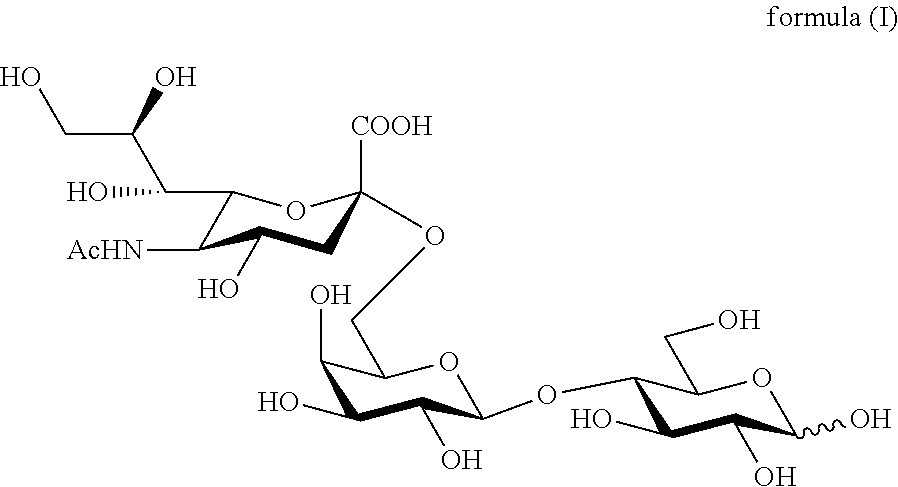
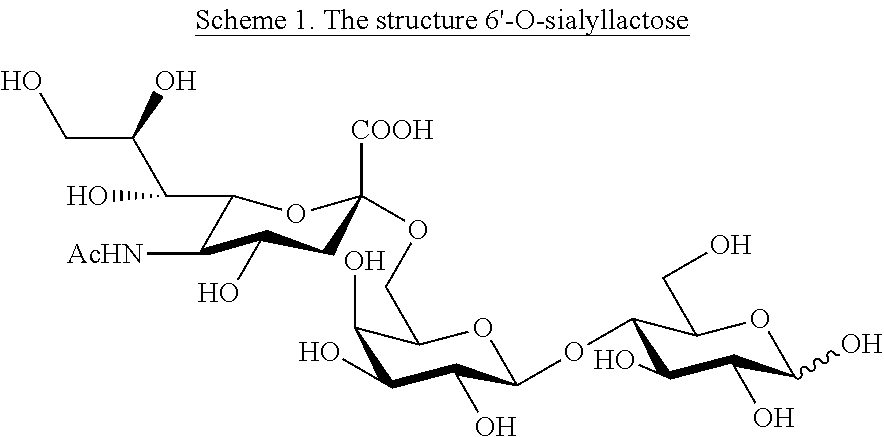
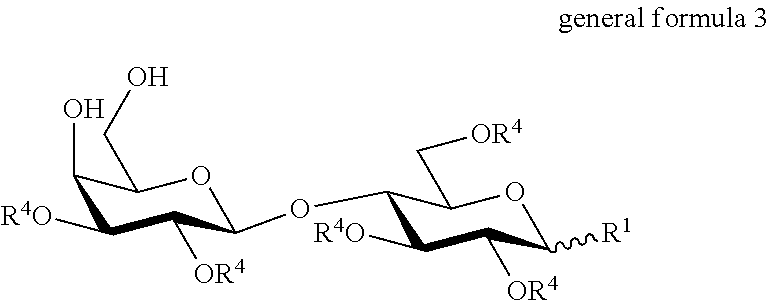
Production of 6'-o-sialyllactose and intermediates
a technology intermediates, which is applied in the field of oligosaccharides and derivatives, can solve the problems of unfavorable steric and electronic effects of stereoselective, inability to access large volumes of sialylated human milk oligosaccharides, and inability to scale up methodologies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0218]
[0219]A mixture of anhydrous sialic acid (100 g, 323 mmol) and dried Amberlite IR-120 (H+) ion exchange resin (100 g) in MeOH (1500 mL) was stirred for 15 hours at RT. The ion exchange resin was filtered off and washed with MeOH (2×100 mL). The washes were combined with the filtrate and concentrated to 300 mL. The concentrated residue crystallized upon seeding at RT. The crystals were collected by filtration giving 73.8 g (71%) sialic acid methyl ester. The mother liquor was concentrated (10.5 g) and recrystallized from MeOH (30 mL) to yield 7.4 g (7%) sialic acid methyl ester. Total yield 81.2 g (78%).
[0220]1H NMR (D2O) δ in ppm: 1.89 (dd, 1H, J=13.0 Hz, J=11.4 Hz); 2.03 (s, 3H); 2.28 (dd, 1H, J=13.0 Hz, J=4.7 Hz); 3.52 (dd, 1H, J=8.9 Hz, J=3.0 Hz); 3.59 (dd, 1H, J=11.6 Hz, J=6.1 Hz); 3.71 (ddd, J=8.9 Hz, J=2.5 Hz, J=11.6 Hz); 3.82 (dd, 1H, J=11.6 Hz, J=2.5 Hz); 3.82 (s, 3H); 3.90 (dd, 1H, J=10.1 Hz, J=10.0 Hz); 3.98-4.10 (m, 2H, H-6, H-4).
example 2
[0221]
[0222]To a suspension of anhydrous sialic acid (100 g, 323 mmol) in MeOH (1200 ml) [0.03% water content] 8% HCl in MeOH (50 ml) was added and the reaction mixture was stirred for 6 hours at RT. The reaction mixture was neutralized with triethylamine (15 ml) and the clear solution was concentrated to 270 mL. The concentrated residue crystallized upon seeding at RT for 2 hours. The solid was collected by filtration yielding 104.9 g (100%).
example 3
[0223]
[0224]A suspension of sialic acid methyl ester (50 g, 155 mmol) and acetic anhydride (73 ml, 775 mmol) in DCM (175 mL) was stirred at RT and 70% perchloric acid (1 mL) was then added dropwise within 30 minutes. During the addition the temperature of the mixture increased until reflux. The reaction mixture was stirred at reflux for 2.5 h, and after this time MeOH (7.5 ml, 185 mmol) was added dropwise and the reaction mixture was stirred for a further hour at RT. The reaction mixture was diluted with DCM (175 mL) and washed with water (3×50 mL). The combined water phases were extracted with DCM (2×100 mL). The combined organic phases were washed with saturated NaHCO3 (2×100 mL) and evaporated. The residue (59.6 g) was dissolved in iBuOAc at 50° C. and the mixture was cooled down to RT and let overnight complete the crystallization. The solid was collected by filtration yielding 39.2 g (52%) of tetraacetyl sialic acid methyl ester.
[0225]1H NMR(C6D6) δ in ppm: 1.60, 1.63, 1.70, 1....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electric charge | aaaaa | aaaaa |
| Electric charge | aaaaa | aaaaa |
| Current | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com