Novel Liposome Nanoparticles for Tumor Magnetic Resonance Imaging
a tumor magnetic resonance imaging and nanoparticle technology, applied in the direction of diagnostic recording/measuring, dispersed delivery, biocide, etc., can solve the problems of low sensitivity, limited mr imaging utility, and difficult formulation of high-concentration liposomes using this lipid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
fifth embodiment
Preferred aspects of this fifth embodiment include:
(31) use according to (30) in the preparation of a magnetic resonance contrast agent for enhancing a magnetic resonance image of a tumour in a mammal.
(32) use according to (30) or (31), wherein the concentration of said liposomes in said magnetic resonance contrast agent is 1-50 mg / mL, more preferably 1-30 mg / mL.
In a sixth aspect of the present invention, there is provided:
(33) a method of magnetic resonance imaging of an organ or organ structure in a mammal, comprising the steps of:
(a) administering the magnetic resonance contrast agent according to (27) or (28) to a patient; and
(b) taking images of the organ of interest in the patient.
sixth embodiment
Preferred aspects of this sixth embodiment include:
(34) a method according to (33), wherein said magnetic resonance contrast agent is used for enhancing a magnetic resonance image of a tumour in a mammal.
(35) a method according to (33) or (34), wherein the concentration of liposomes in said magnetic resonance contrast agent is 1-50 mg / mL, more preferably 1-30 mg / mL.
In a seventh aspect of the present invention, there is provided:
(36) a method of magnetic resonance imaging of an organ or organ structure in a mammal pre-administered with the magnetic contrast agent according to (27) or (28) comprising the step of:
(i) taking images of the organ of interest in the patient.
In an eighth aspect of the present invention, there is provided:
(37) a method of making a liposome according to (1) to (26) comprising mixing a solution of Gd.DOTA.DSA (gadolinium(III)2-14,7-bis-carboxymethyl-10-[(N,N-distearylamidomethyl-N′-amido-methyl]-1,4,7,10-tetra-azacyclododec-1-yl}-acetic acid) and a solution of...
eighth embodiment
A preferred aspect of the eighth embodiment includes:
(38) drying the mixture (e.g. in vacuo) and optionally rehydrating the resulting liposome.
In a ninth aspect of the present invention, there is provided:
(39) a method of making a magnetic contrast agent according to (27) or (28) comprising mixing a liposome of (1) to (26) and a pharmaceutically acceptable carrier.
Preferred aspects of the eighth and ninth embodiments are the same as those listed above in relation to the first, second and third aspects.
DETAILED DESCRIPTION OF THE INVENTION
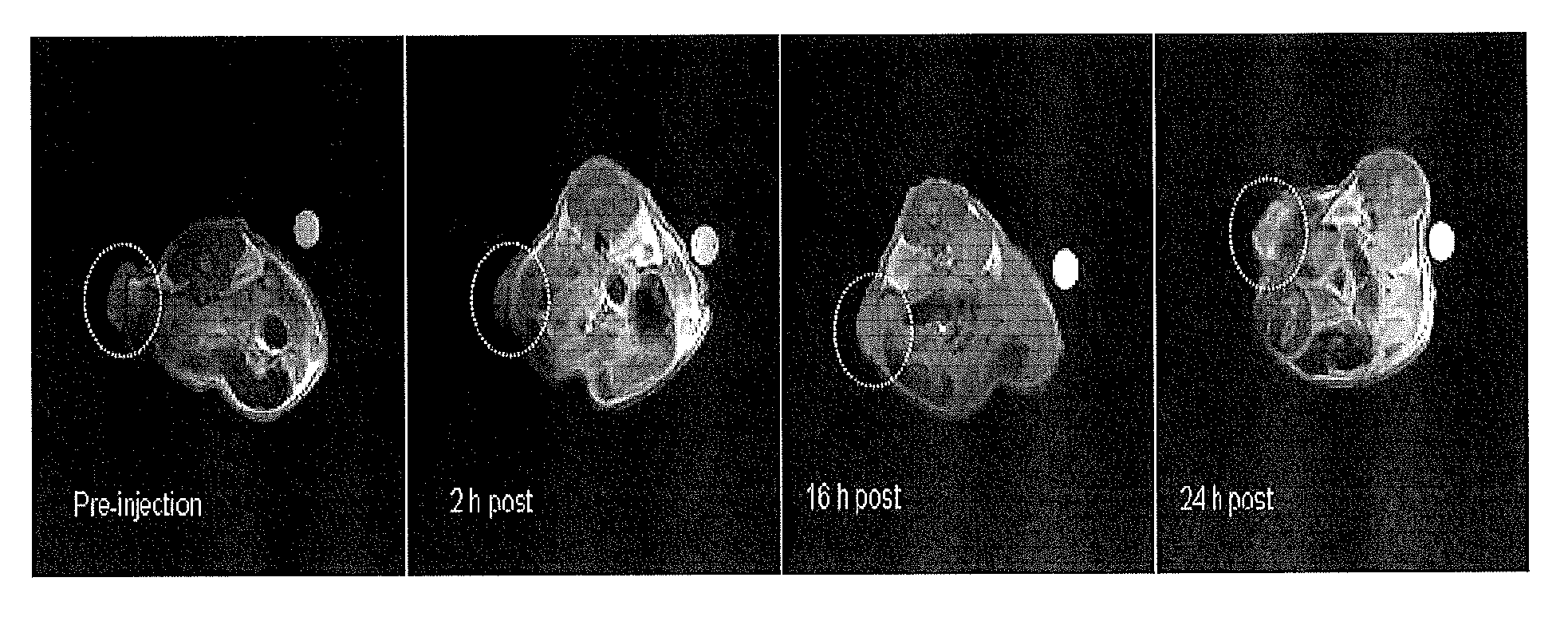
[0020]We will now discuss the present invention in further detail. The present invention may also be further understood by reference to FIGS. 1 to 29, wherein:
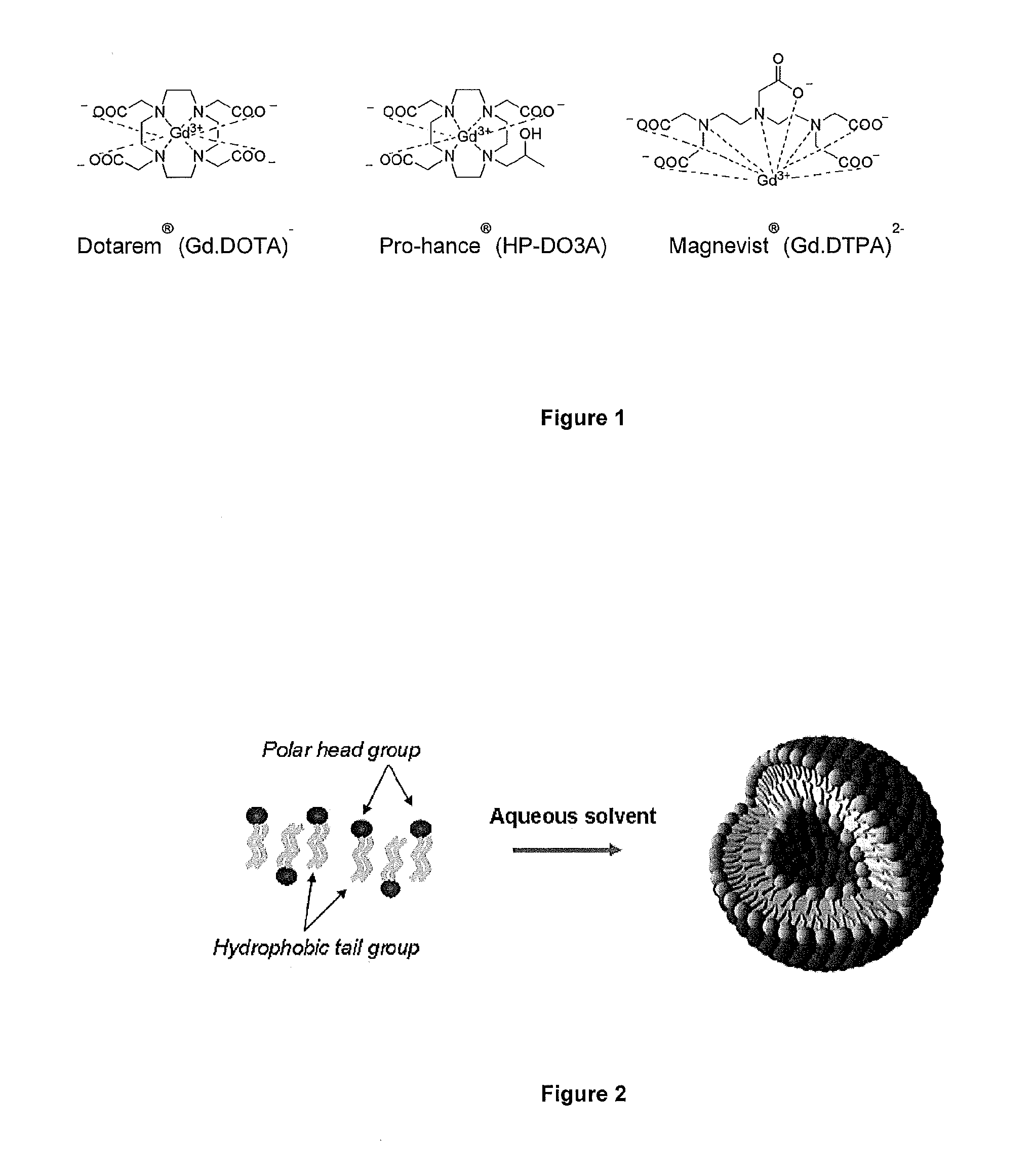
[0021]FIG. 1 shows Gadolinium based clinical contrast agents approved by the FDA;
[0022]FIG. 2 shows liposome formation from amphipathic lipids;
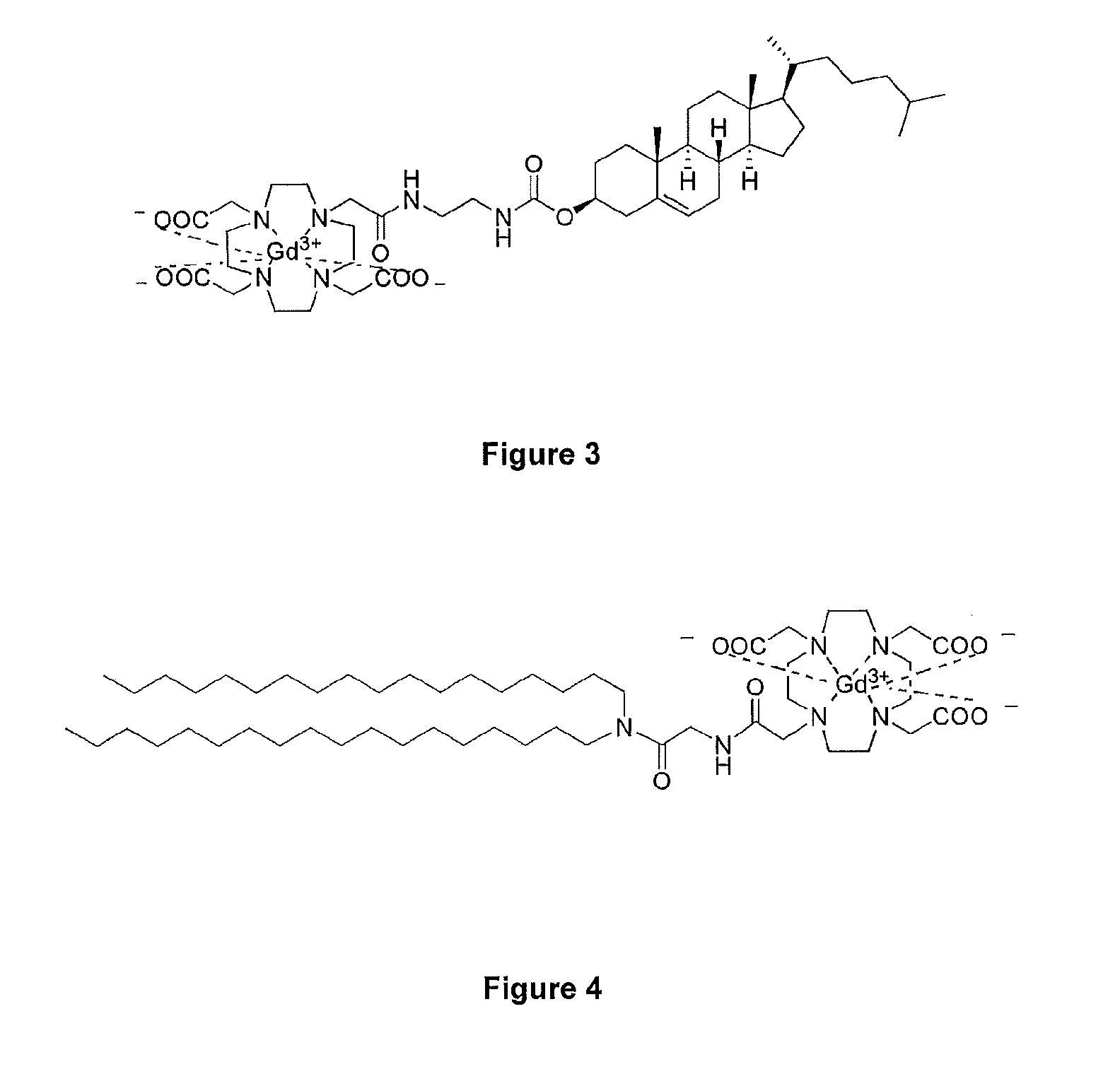
[0023]FIG. 3 shows Gd.DOTA.Chol, a T1 lipidic contrast agent component of MAGfect;
[0024]FIG. 4 shows the paramagnetic gadolinium lipid target, Gd.DOTA....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com