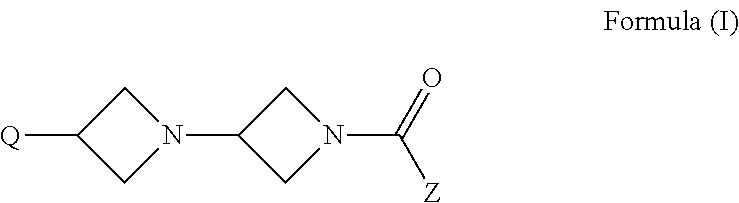
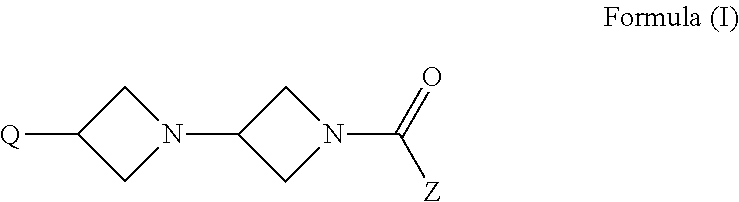
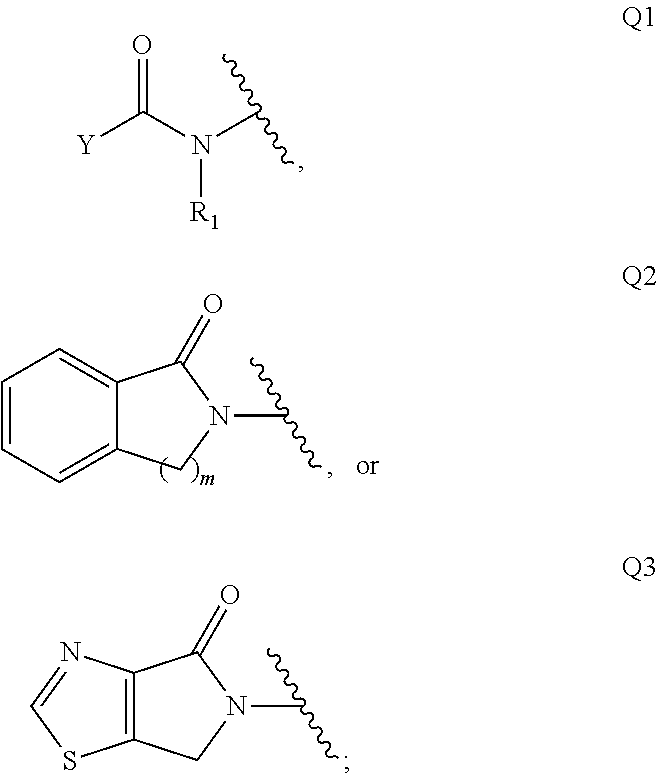
Di-azetidinyl diamide as monoacylglcerol lipase inhibitors
a technology of diazetidinyl diamide and monoacylglcerol, which is applied in the direction of biocide, drug composition, animal repellent, etc., can solve the problem of difficult to separate the beneficial effects of these compounds from the unwanted side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 5
[0216]
[0217]2-{1′-[(6-Phenylnaphthalen-2-yl)carbonyl]-1,3′-biazetidin-3-yl}-2,3-dihydro-1H-isoindol-1-one, Cpd 69
[0218]A mixture of compound 90 (40 mg, 0.08 mmol), phenylboronic acid (21 mg, 0.17 mmol), K2CO3 (23 mg, 0.17 mmol), and Pd(dppf)Cl2.CH2Cl2 (4 mg, 0.005 mmol) in EtOH (0.75 mL) and H2O (0.15 mL) was heated in a microwave reactor at 130° C. for 30 min. The mixture was diluted with CH2Cl2, washed with H2O, dried over Na2SO4 and concentrated. Purification by flash column chromatography (silica gel, 3% MeOH / CH2Cl2) gave compound 69 (34 mg). MS 474 (M+H+).
[0219]Following the procedure described above for Example 5 and substituting the appropriate reagents, starting materials and purification methods known to those skilled in the art, the following compounds of the present invention were prepared:
CpdName and data2N-(1′-{[3′-(Trifluoromethyl)biphenyl-4-yl]carbonyl}-1,3′-biazetidin-3-yl)-1,3-thiazole-4-carboxamide.1H NMR (CHLOROFORM-d, 400 MHz): δ = 8.77 (s, 1H), 8.18 (d, J = 2.4 ...
example 6
[0220]
A. tent-Butyl 3-(benzyl(methyl)amino)azetidine-1-carboxylate, 6b
[0221]To a solution of 1-Boc-azetidin-3-one 1d (1.0 g, 5.85 mmol) and N-methyl-benzylamine 6a (1.02 g, 8.43 mmol) in 1,2-dichloroethane (12 mL) and acetic acid (1 mL) was added Na(OAc)3BH (1.30 g, 6.13 mmol). The reaction mixture was stirred at room temperature for 20 h. The reaction was quenched by the addition of aq. NaHCO3. The resulting mixture was extracted with CH2Cl2. The organic solution was dried over Na2SO4 and concentrated. Purification by flash column chromatography (silica gel, 40% EtOAc / heptane) gave compound 6b (1.40 g).
B. tent-Butyl 3-(benzyl(methyl)amino)-[1,3′-biazetidine]-1′-carboxylate, 6d
[0222]Intermediate 6d was prepared following the procedure described in Step B of Example 1.
C. (3-(Benzylmethyl)amino)-[1,3′-biazetidin]-1′-yl)(6-(trifluoromethyl)benzo[b]thiophen-2-yl)methanone, 6f
[0223]Intermediate 6f was prepared following the procedure described in Example 4.
D. N-Methyl-N-(1′-{[6-(trifluor...
examples 7-28
provide synthetic routes to useful intermediates for the preparation of compounds of Formula (I).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com