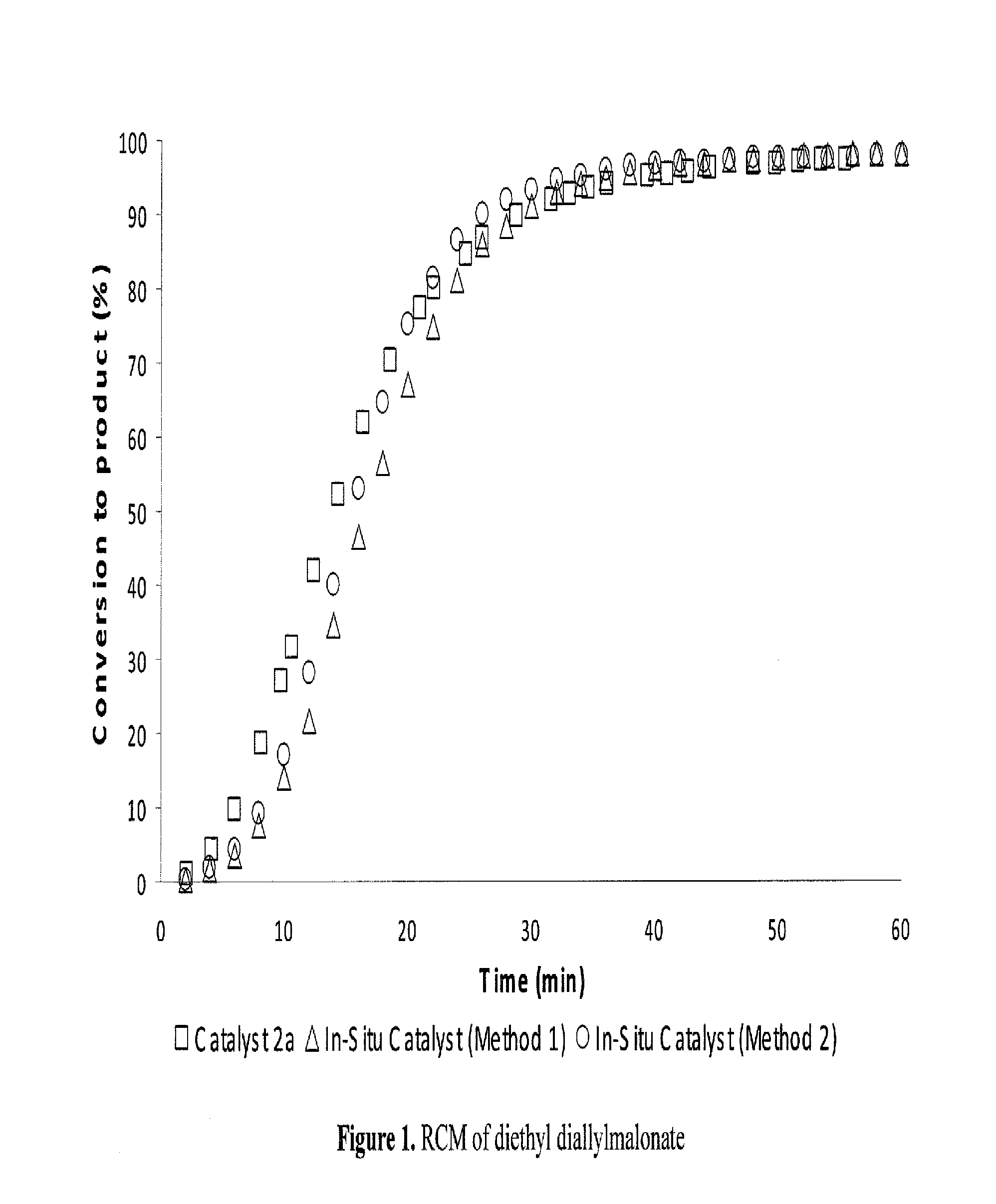
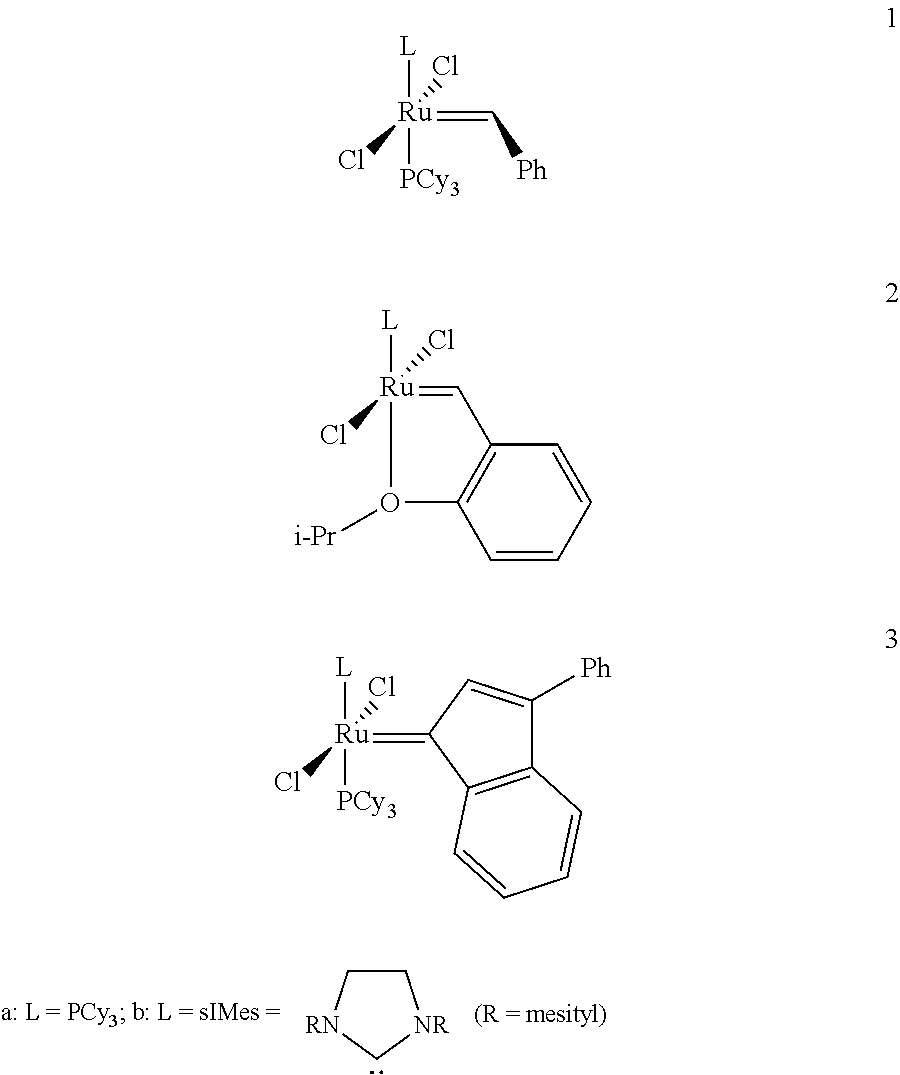
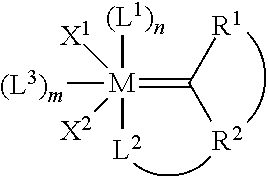Method for in-situ formation of metathesis catalysts
a technology of organic olefin and catalyst, which is applied in the direction of organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, ruthenium organic compound, etc., can solve the problems of less active types of complexes and relatively cumbersome syntheses of these complexes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of 3,5-dimethoxybenzophenone
[0096]
[0097]This compound was previously prepared by a two-step process.v However, we prepared it by a one-step method adapted from another literature procedure.vi Neat 3,5-dimethoxybenzonitrile (16.0 g, 98.1 mmol) was added to a 2.0 M solution of phenylmagnesium chloride in THF (98.0 mL, 196.1 mmol). The reaction mixture was refluxed for 24 hours at 70° C. The solution was then transferred into a mixture of concentrated aqueous HCl (100 mL) and ice (300 g). The mixture was allowed to warm up to room temperature and stirred for 24 hours. The product was extracted with ether (3×300 mL) and the combined organic layers were washed with brine (200 mL) and water (150 mL) before being dried with anhydrous magnesium sulfate. The filtrate was dried in vacuo to afford 3,5-dimethoxybenzophenone as a yellow solid in 75% yield. 1H NMR (CDCl3): δ 7.84 (d, 3JH—H=7.2 Hz, 1H), 7.61 (t, 3JH—H=7.6 Hz, 2H), 7.50 (t, 3JH—H=7.6 Hz, 2H), 6.95 (d, 3JH—H=2.4 Hz, 1H), 6...
example 1a
Synthesis of 3,5-diisopropoxybenzophenone
[0098]
[0099]3,5-Diisopropoxybenzophenone was prepared from 3,5-diisopropoxybenzonitrile following a procedure analogous to that described above for the preparation of 3,5-dimethoxybenzophenone (Example 1). 3,5-Diisopropoxybenzonitrile was synthesized according to a literature procedure (Wang, E.-C.; Lin, G.-J. A New One Pot Method for the Conversion of Aldehydes into Nitriles Using Hydroxyamine and Phthalic Anhydride. Tetrahedron Lett. 1998, 39, 4047-4050).
example 2
Synthesis of 1-(3,5-Dimethoxyphenyl)-1-Phenylprop-2-yn-1-ol
[0100]
[0101]The following method was adapted from a literature procedure.vii Anhydrous THF (50 mL) was cooled to −78° C. Purified acetylene gas was gently bubbled through the THF for 1 hour. A 2.5 M solution of n-butyllithium in THF (8.2 mL, 20.5 mmol) was then added drop-wise and the mixture was stirred vigorously for 20 minutes. 3,5-Dimethoxybenzophenone (5.0 g, 20.5 mmol) was dissolved in 10 mL of anhydrous THF and the solution was slowly dropped into the reaction flask. The mixture was stirred vigorously for 20 minutes at −78° C. before being allowed to slowly warm to room temperature. A 5% aqueous solution of NH4Cl (60 mL) was added and the mixture stirred for 30 minutes. The product was extracted with ether (3×100 mL) and the combined organic layers were washed with brine (100 mL) and water (75 mL) before being dried with anhydrous magnesium sulfate. The filtrate was dried in vacuo to afford 1-(3,5-dimethoxyphenyl)-1-p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com