Methods and compositions for the improvement of skeletal muscle function in a mammal
a skeletal muscle and composition technology, applied in the field of mammalian skeletal muscle function improvement methods and compositions, can solve the problems of no established clinical regimen for treating muscular dysfunction, and achieve the effects of reducing muscle fatigue, improving ability, and reducing muscle fatigu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Metformin Increases Strength and Reduces the Fatigue Rate of Mouse Skeletal Muscle Tissue from a Genetic Model of Duchenne Muscular Dystrophy
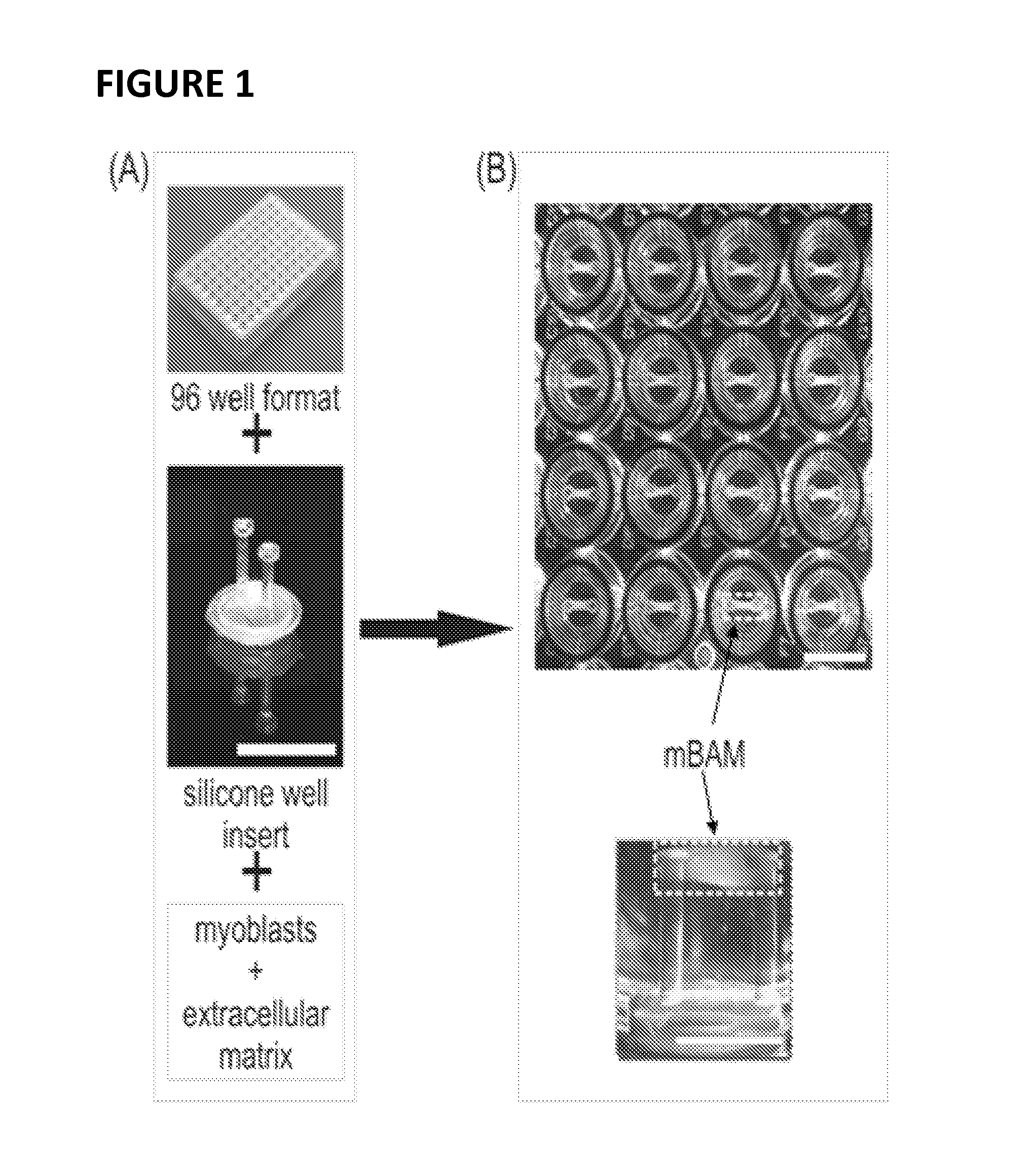
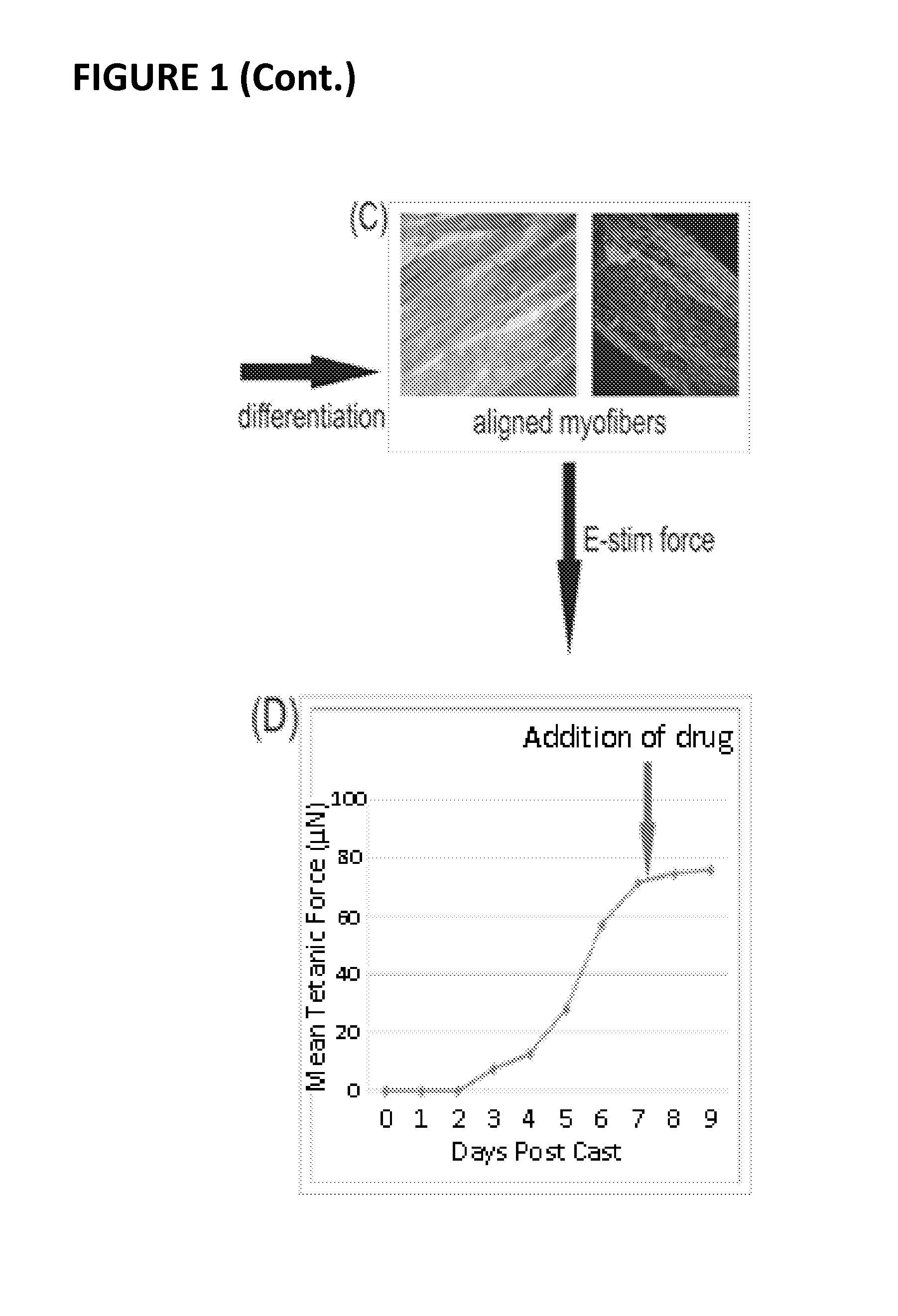
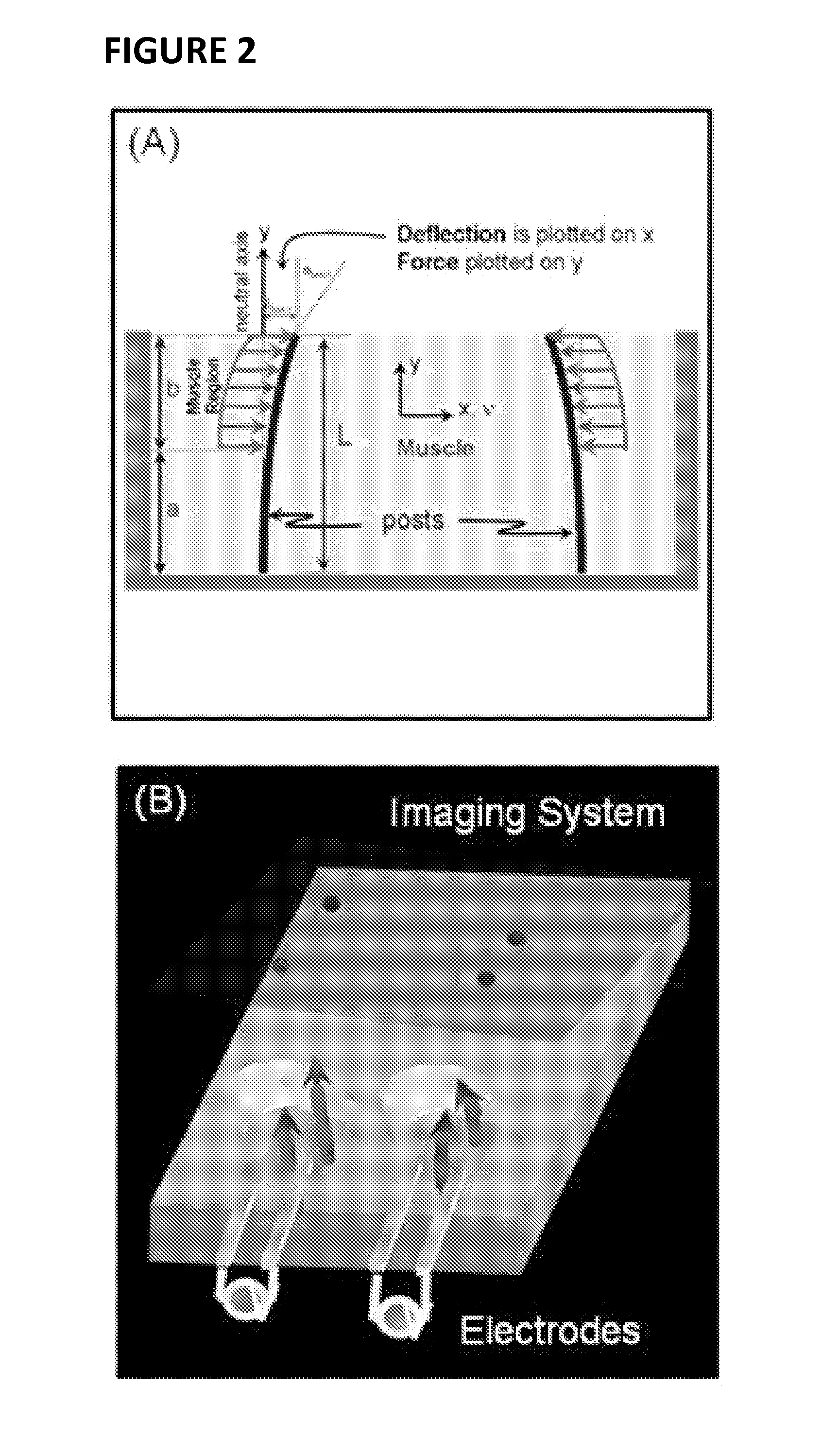
[0115]Conditionally immortalized skeletal muscle cells from the mdx mouse muscle (Morgan et al., Dev Biol. 162: 486-498, 1994) were tissue engineered into contractile muscle tissue (mBAMs), as described herein. (See also, e.g., Vandenburgh et al., Muscle and Nerve 37: 438-447, 2008; Vandenburgh et al., Treat NMD Meeting, 2009). Briefly, several hundred thousand proliferating myoblasts were mixed with a solution of extracellular matrix (collagen or fibrin) and pipetted into custom wells containing two flexible “posts” (FIG. 1A). The cell / matrix mixture gelled around the tops of the posts, forming a defined tubular structure (FIG. 1B). The myoblasts differentiated into several hundred organized contractile muscle fibers (called miniature BioArtificial Muscle (mBAM); FIG. 1C) in tissue culture medium, such as that described in Vandenburgh et al., ...
example 2
Biguanides Increase Skeletal Muscle Strength and Decrease the Rate of Muscle Fatigue of Normal Mouse Skeletal Muscle Tissue
[0118]Skeletal muscle cells from the leg muscles of normal mice were isolated by standard tissue culture protocols (Rando et al., J Cell Biol. 125: 1275-1287, 1994), tissue engineered into contractile muscle tissue (mBAMs), and muscle force and fatigue measured as described in Example 1 (above).
[0119]High glucose tissue culture medium (4.5 g / l) was used in all experiments to maintain glucose levels well above normal human blood plasma levels (0.9 to 1.8 g / l) and, thereby, minimize drug action through increased glucose availability to the muscle cells. On Days 6-8 post-casting, metformin, phenformin (Fluka Cat. No. P7045-1G), or proguanil (Ipca Laboratories, CAS No. 637-32-1, Batch No. 8001HPRI) at different concentrations were added to the tissue culture medium, and maximal tetanic force was measured 24 hours later in a MFAS™. Every 24 hours, fresh metformin, ph...
example 3
Biguanides Increase Skeletal Muscle Strength and Reduce the Rate of Muscle Fatigue of Normal Human Muscle Tissue
[0123]Skeletal muscle cells from the vastus lateralis muscle of human volunteers were isolated by thin needle muscle biopsy and grown by standard tissue culture protocols (Shansky et al., “Tissue engineering human skeletal muscle for clinical applications” in Culture of Cells for Tissue Engineering (G. Vunjak and I. Freshney, eds.), pages 239-257, 2006), tissue engineered into contractile muscle tissue (mBAMs), and muscle force and fatigue measured as described in Example 1. On Days 7-10 post-casting, metformin, phenformin, buformin, or proguanil at different concentrations were added to the tissue culture medium and maximal tetanic force measured 24 hours later in MFAS™. High glucose tissue culture medium (4.5 g / l) was used to maintain glucose levels well above normal human blood plasma levels (0.9 to 1.8 g / l) and, thereby, minimize drug action through increased glucose a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com