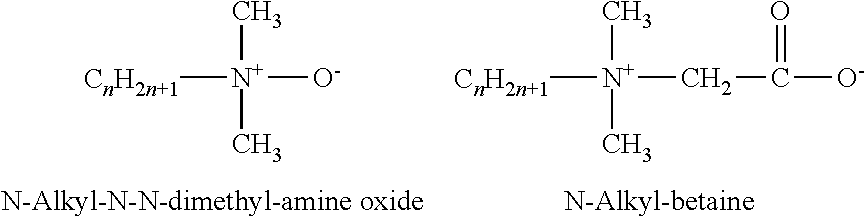Green Glycine Betaine Derivative Compounds And Compositions Containing Same
a technology of green glycine betaine and derivative compounds, which is applied in the direction of detergent compounding agents, ampholytes/electroneutral surface active compounds, biocides, etc., can solve the problem that the patent does not disclose the preparation of gb or derivatives of gb from natural sources
Inactive Publication Date: 2013-12-19
SAINT VICTOR MARIE ESTHER +2
View PDF8 Cites 17 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
The invention is about creating sustainable, multi-functional, green compositions for cleaning, gels, aerosols, and wipes. These compositions use natural and green ingredients that provide both antimicrobial and surfactant properties. The natural ingredients are effective against a wide range of bacteria and fungi, and can be used as adjuvants in pesticide and herbicide applications. The compositions also help to control the delivery of fragrances and essential oils. The invention is particularly about glycine betaine components, which are cationic and positively charged rather than being zwitterionic or amphoteric. These components exhibit antimicrobial activities similar to quaternary ammonium salts and can be very effective adjuvants in agricultural formulations. The glycine betaine derivatives also play multiple functions in compositions, reducing the need for additional cleaning or wetting agents. The ability of the glycine betaine-based surfactants to emulsify vegetable oils is also demonstrated. The patent describes a method for preparing betainyl amino alkyl methane sulfonates, which are used in the compositions.
Problems solved by technology
This patent does not disclose preparation of GB or derivatives of GB from natural sources.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
example i
[0090]Application Bath Dodecyl / Tetradecylbetainate methanesulfonate Ester C12 / C14 Pure as surfactant and antimicrobial agent.
example ii
[0091]Application Bath Dodecyl / Tetradecylbetainate methanesulfonate Ester C12 / C14 crude as surfactant and antimicrobial agent.
example iii
[0092]Application Bath (Z)-Betainylaminooctadec-9-ene methanesulfonate C18:1 as surfactant and antimicrobial agent.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Login to View More
Abstract
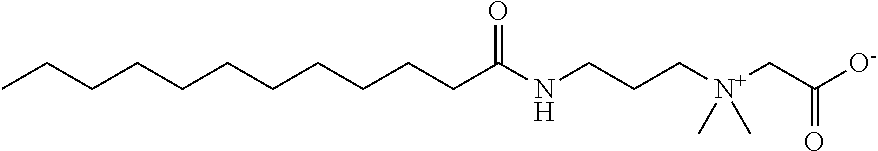
Multifunctional green (eco-friendly) and antimicrobial compositions are described containing cationic glycine betaine esters and / or cationic glycine betaine amides. Particular glycine betaine esters and amides are alkyl(ene) betainate methane sulfonates and betainyl amino alkyl(ene) methane sulfonates. The glycine betaine components are cationic, have a hydrophobic group attached to a carboxylate group through an ester or amide linkage, and are derived from a natural source, such as sugar beets. The glycine betaine esters and amides serve as cationic surfactants which have effective antimicrobial activity. The surfactant compositions are effective as crude mixtures or semi-purified mixtures or purified surfactant compounds of glycine betaine components. The addition of sodium chloride or potassium chloride or magnesium chloride or natural gum or polysaccharide to compositions containing the cationic glycine betaine ester and / or glycine betaine amide derivatives serves to thicken or gel the composition.
Description
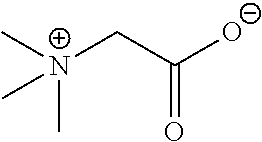
FIELD OF INVENTION[0001]Green (eco-friendly) and multifunctional cationic glycine betaine derivative compounds having surfactant and antimicrobial properties, in particular ester and amide derivatives, including alkyl(ene) betainate methane sulfonates and betainyl amino alkyl(ene) methane sulfonates, in semi-pure and crude mixtures and pure form, are described. Such compounds are includable in various household compositions to optimize solubilization, wetting, cleaning, emulsification of various oils and soils, and to provide effective antimicrobial properties. Further modification of compositions containing such derivatives is also provided through combination of the glycine betaine derivative(s) and sodium chloride to obtain thickening or gelling of the composition.BACKGROUND OF THE INVENTION[0002]Glycine betaine (GB) (Formula 1 below) is a natural and inexpensive product derived from sugar beet molasses and constitutes a prime raw material for the preparation of biodegradable and...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(United States)
IPC IPC(8): A01N37/44C11D3/60C07C309/04C07C303/32C07C229/08A01P1/00C07C227/18
CPCC11D1/90A01N25/30A01N37/44C11D3/222C11D3/48
Inventor SAINT VICTOR, MARIE-ESTHERBENVEGNU, THIERRYAZIRA, HAKIMA-FATIMA
Owner SAINT VICTOR MARIE ESTHER
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com