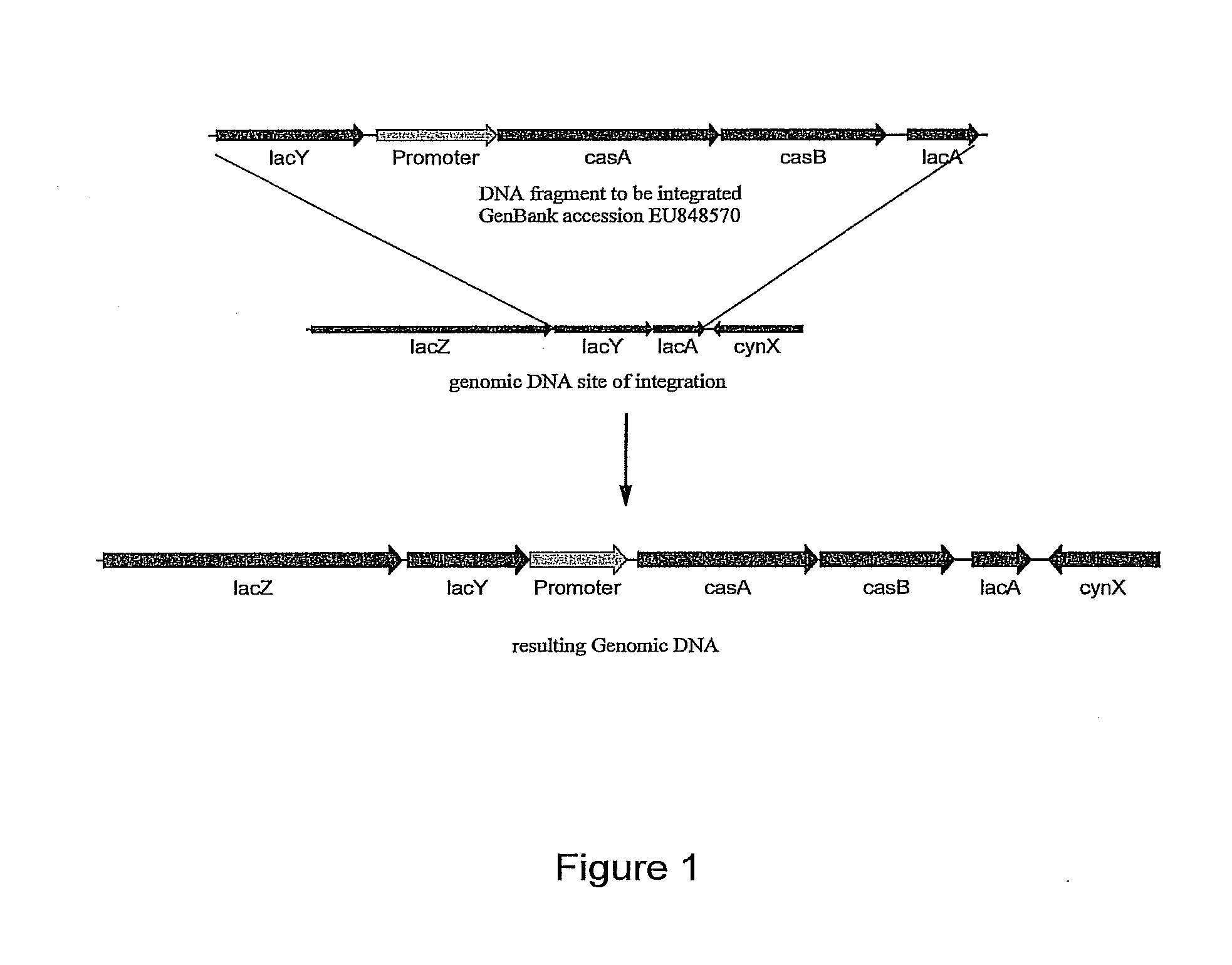
Methods and compositions for degrading pectin
a technology of pectin and composition, applied in the field of methods and compositions for degrading pectin, can solve the problems of pectin being esterified or unesterified,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
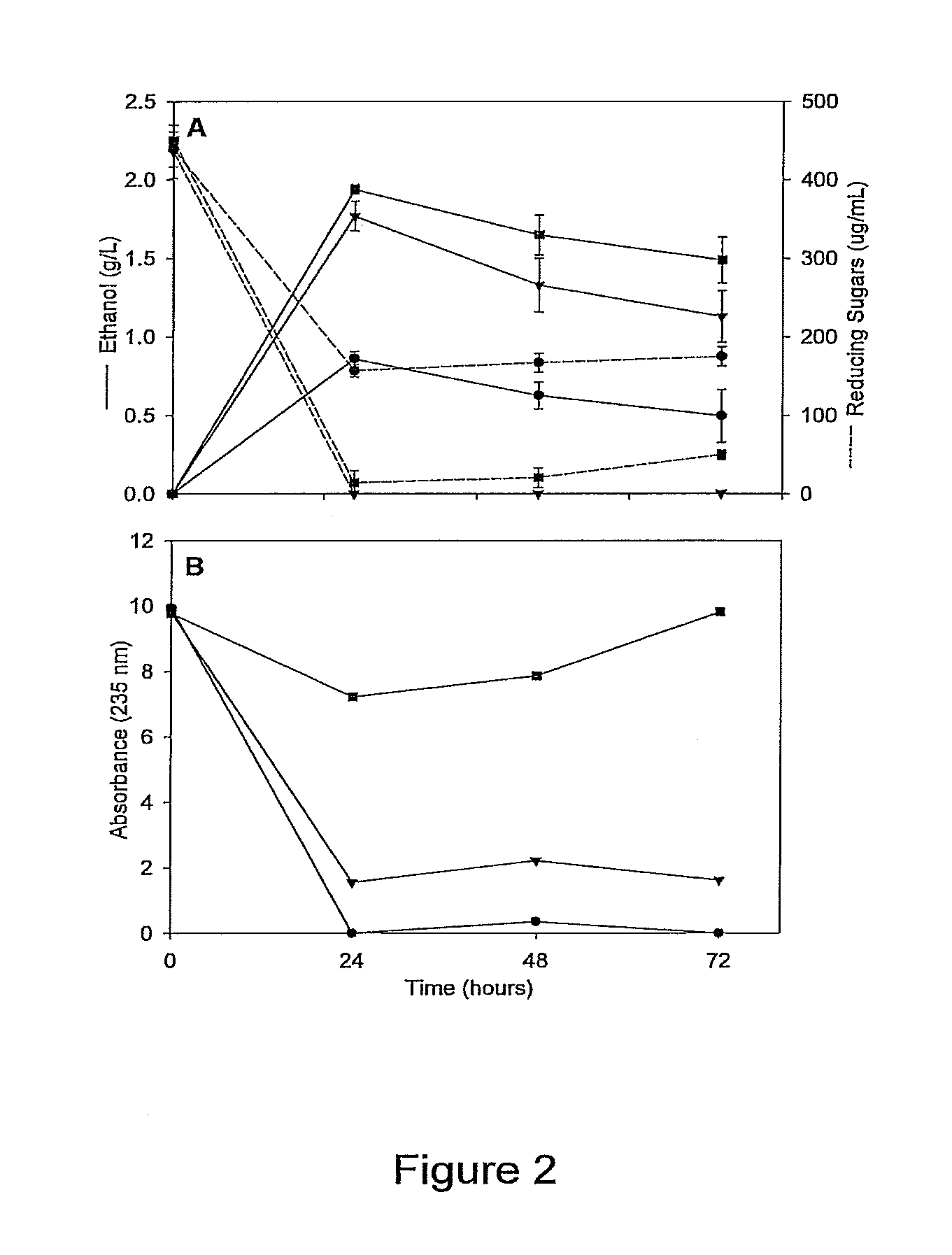
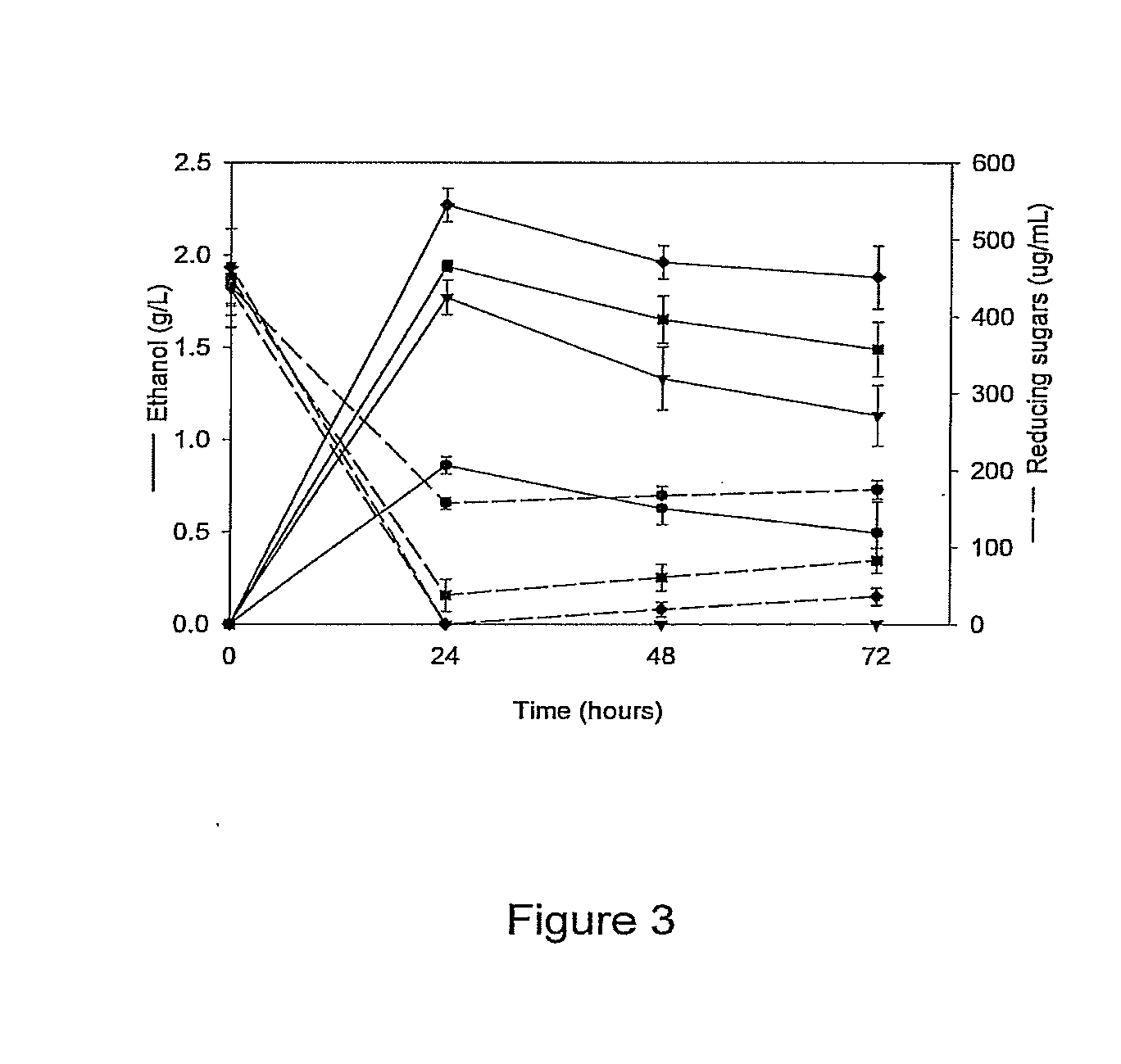
[0102]Ethanologen Escherichia coli KO11 was sequentially engineered to produce the Klebsiella oxytoca EnzymeIIcellulose and phospho-βglucosidase genes (casAB) as well as a pectate lyase (pelE) from Erwinia chrysanthemi, yielding strains LY40A (casAB) and JP07 (casAB; pelE), respectively. To obtain effective secretion of PelE, the Sec-independent pathway out genes from E. chrysanthemi on the cosmid pCPP2006 were provided to strain JP07 to construct strain JP07C. E. coli strains LY40A, JP07, and JP07C possessed significant cellobiase activity in cell lysates, while only strain JP07C demonstrated extracellular pectate lyase activity. Fermentation with sugar beet pulp at very low fungal enzyme loads during saccharification revealed significantly higher ethanol production for LY40A and JP07C compared to KO11. While JP07C ethanol yields were not considerably higher than LY40A, investigation of oligogalacturonide polymerization showed an increased breakdown of biomass to small chain (degre...
example 2
[0123]Paenibacillus amylolyticus C27 was isolated from the hindgut of Tipula abdominalis and found to produce lignocellulose-degrading enzymes. A library was constructed with C27 genomic DNA for heterologous expression of biological characteristics in Escherichia coli. Two pectate lyase genes, pelA and pelB, were identified while screening a genomic library in E. coli for pectinase activity. PelA encodes a 222 amino acid protein and demonstrated highest activity on polygalacturonic acid, but retained 60% and 56% of maximum activity on 8.5% and 90% methylated pectin, respectively. CaCl2 was required for activity, and optima were pH 10.5, 45° C., and 1.5 mM CaCl2. PelA has high identity (95%) to PelA from P. barcinonensis, and is a subclass of the pectate lyase family III from saprophytic, non-pathogenic bacteria. On the other hand, pelB encodes a 392 amino acid protein. Although PelB showed the highest activity on 20-34% methylated pectin, it retained 67%, 51%, 25%, and 1% of its max...
example 3
[0144]Degradation of sugar beet pulp and examination of oligogalacturonides. Precultures of E. coli carrying plasmid pEDH27 were grown in LB with 50 mg L−1 ampicillin overnight with shaking at 37° C. and inoculated into LB with 5% dry wt L−1 sugar beet pulp to OD550 0.5. Sugar beet pulp cultures were grown at 37° C. with shaking and samples were removed every 24 h.
[0145]To quantify oligogalacturonides with a degree of polymerization (dp) less than 6, fermentation supernatant was diluted 1:3 in water and ethanol was added to a final concentration of 11% (vol / vol). The solution was incubated with agitation for 16 h at 4° C. and then centrifuged at 7500 g for 15 min. This supernatant was diluted and analyzed at 235 nm (Spiro et al., 1993, Carbohydr. Res., 247:9-20) and compared to the absorbance of fermentation supernatant preparation immediately after inoculation.
[0146]Construction of E. coli JP27. Plasmid pEDH27 was transformed by heat shock into E. coli LY40A to construct strain JP2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com