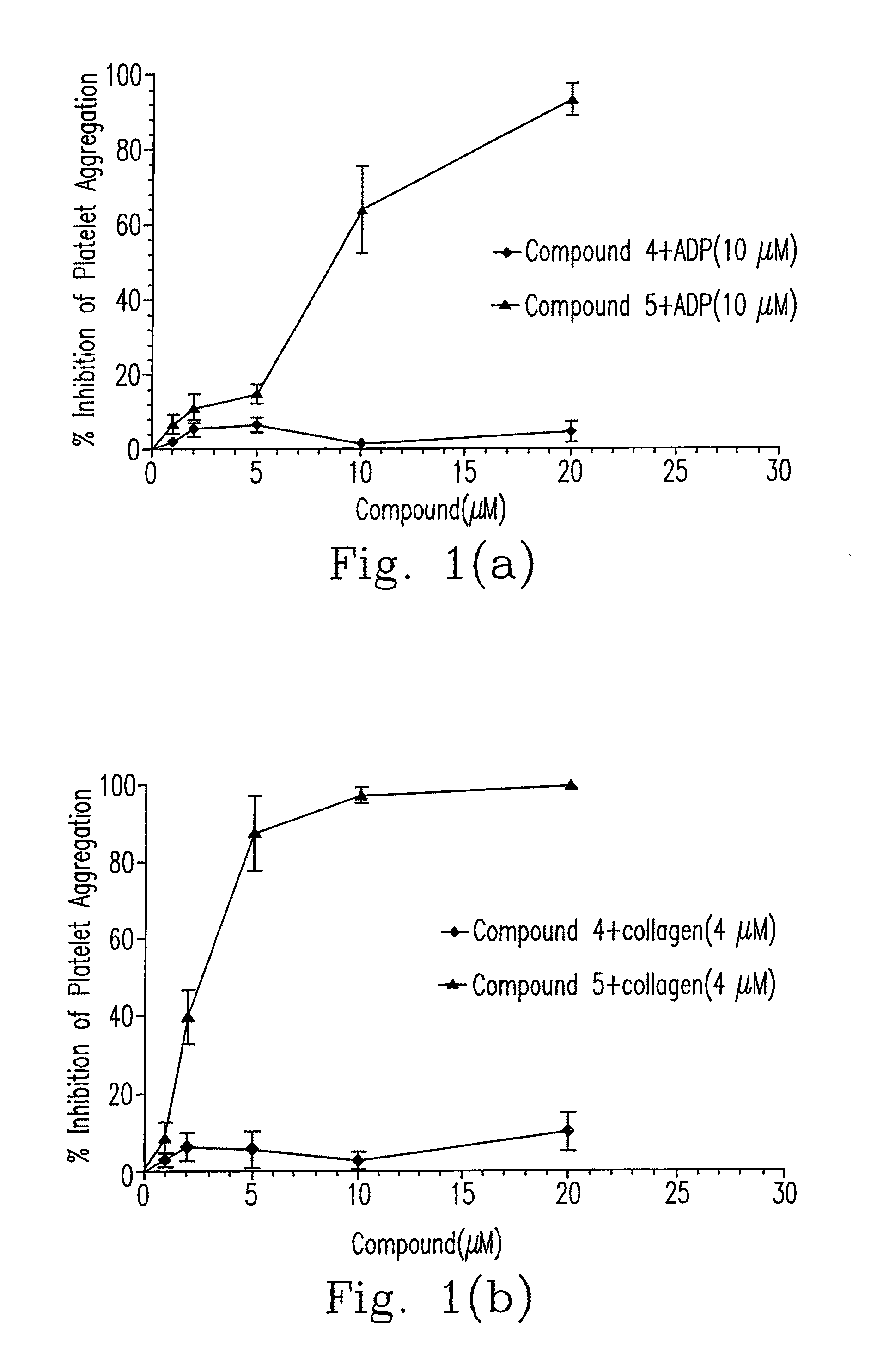
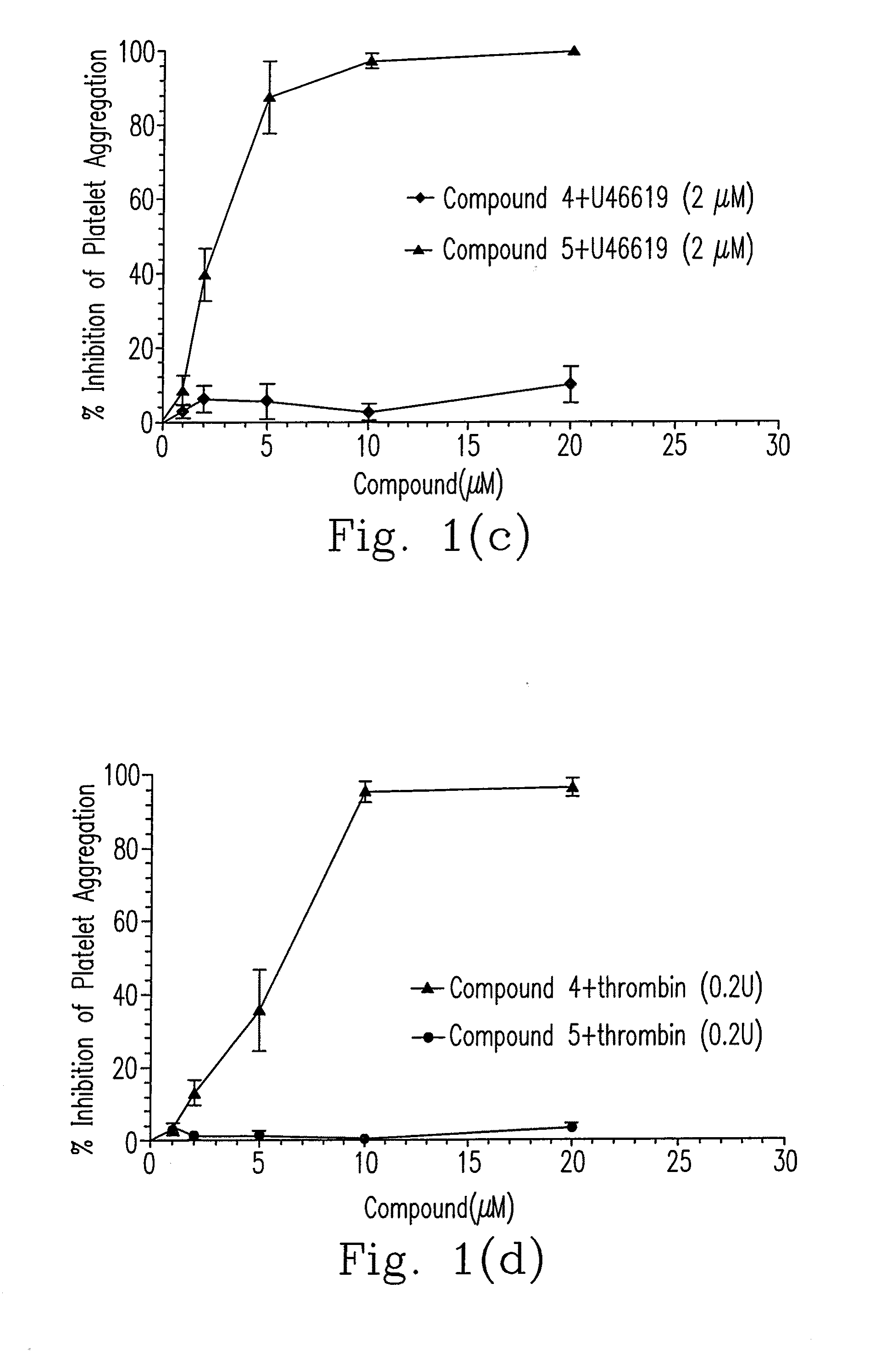
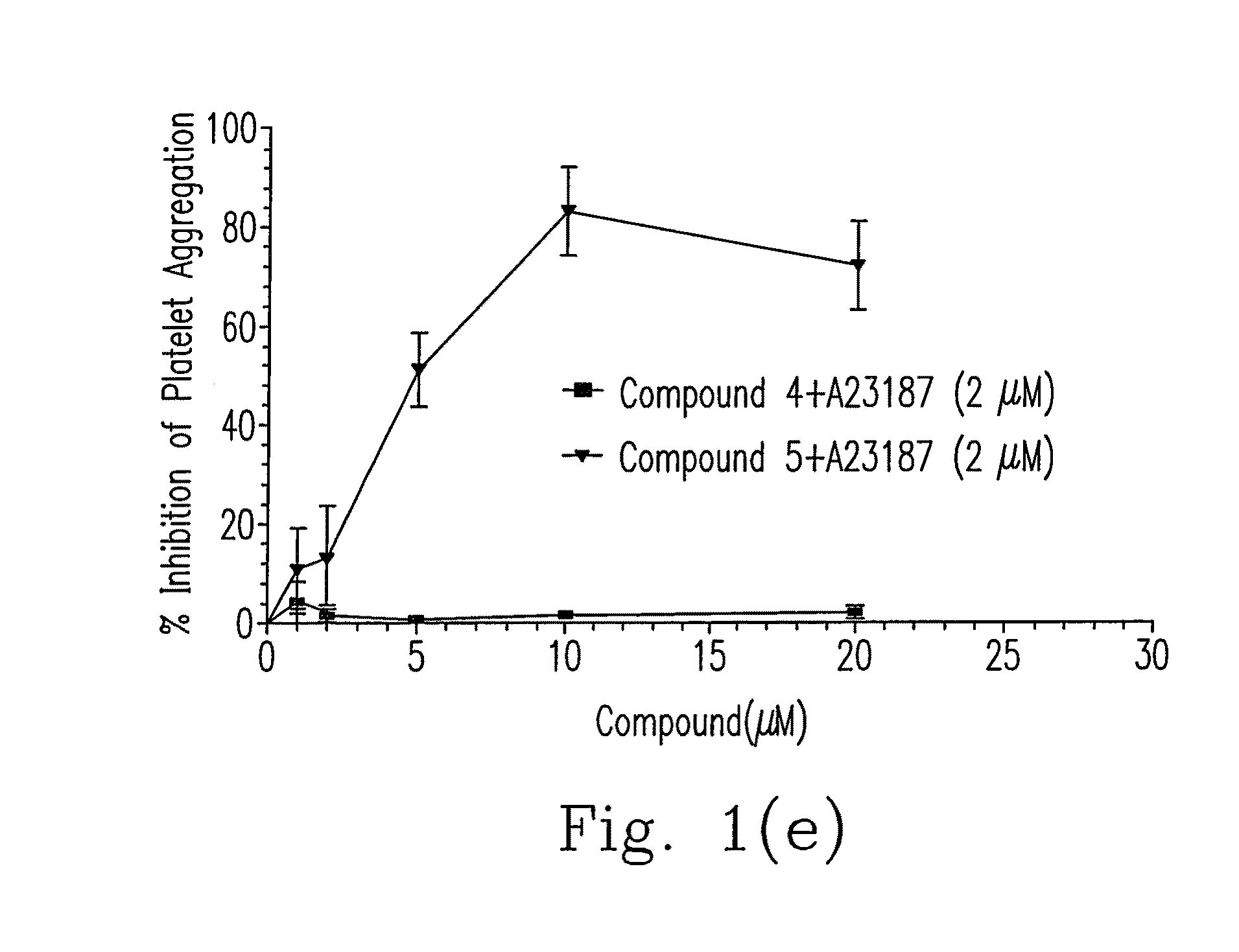
Composition of 5-nitrobenzoate derivatives as Anti-metastatic agent that inhibits tumor cell-induced platelet aggregation
a technology of tcipa and derivatives, which is applied in the field of 5nitrobenzoate derivatives, can solve the problems of inability to show specificity, inability to inhibit aspirin, and complicated details of tcipa, and achieve the effect of inhibiting tcipa and inhibiting platelet aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
of N-(2-benzamidoethyl)-2-hydroxy-5-nitrobenzamide
[0028]The preparation procedure of N-(2-benzamidoethyl)-2-hydroxy-5-nitrobenzamide is represented by the following formula II.
[0029]1-Chloro-4-nitro-2-(trifluoromethyl)benzene (2.0 mL, 13.3 mmole) was dissolved in dimethyl sulfoxide (DMSO, 12 mL), NaOH (1.6 g) was batchwise added at a temperature lower than 25° C., and the reaction solution was reacted at room temperature (RT) for 8 hours. After the reaction was terminated, the pH of the reaction solution was adjusted to 1.0 using concentrated HCl, and then the reaction solution was poured into the separatory funnel and extracted with CH2Cl2 for five times (each for 20 ml). The obtained CH2Cl2 solution was hydrated over MgSO4 and concentrated under vacuum. The obtained concentrate was subjected to the purification of silica gel column (50 g) and eluted with the system of CHCl3 / n-hexane (2:1) to afford compound 1 (1.85 g), yield of about 67%.
[0030]Compound 1: 1H NMR (400 MHz, CDCl3): ...
embodiment 2
of Other 5-nitrobenzoate Derivatives
[0041]For affording other 5-nitrobenzoate derivatives, compound 3 may be reacted with benzoyl chlorides bound with a various of substituted groups, such as mono-substituted benzoyl chloride, di-substituted benzoyl chloride or tri-substituted benzoyl chloride
respectively), and each of R1, R2, R3, R4, R5 and R6 may be fluoride, chloride, bromide, iodide or methyl group, and R1 to R6 may be bound to the para-, meta- or ortho-position of the benzoyl moiety. That is, the benzoyl moiety of the prepared compound 4 may be substituted as mono-substituted benzoyl moiety, di-substituted benzoyl moiety or tri-substituted benzoyl moeity
respectively).
embodiment 3
of 4-O-benzoyl-3-methoxy-β-nitrostyrene (compound 5)
[0042]trans-4-Hydroxyl 3-methoxyl-β-nitrostyrene and benzoyl chloride, were dissolved in a mixture solution of pyridine (1 ml) and CH2Cl2 (10 ml), and reacted at RT for 24 hours. After the removal of solvent, the obtained concentrate was subjected to the purification of silica gel column (90 g) and eluted with the system of n-hexane / acetone (3:1) to afford 4-O-benzoyl-3-methoxy-β-nitrostyrene (compound 5; as represented by formula III).
[0043]Compound 5: 1H NMR (400 MHz, CDCl3): δ 8.25 (1H, s), 8.24 (1H, s), 8.02 (1H, d, J=13.6 Hz), 7.68(1H, d, J=7.6 Hz), 7.61(1H, d, J=13.6 Hz), 7.56(1H, d, J=7.6 Hz), 7.55(1H, d, J=7.6 Hz), 7.28(1H, d, J=8.2 Hz), 7.24(1H, d, J=8.2 Hz), 7.17 (1H, d , J=1.2 Hz), 3.88 (3H, s). ESI-MS m / z 322 (100) [M+Na]+.
[0044]Compound 5 was nominated as 4-O-benzoyl-3-methoxy-β-nitrostyrene).
PUM
| Property | Measurement | Unit |
|---|---|---|
| aggregation | aaaaa | aaaaa |
| tumor cell-induced platelet aggregation | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com